III. Amino Sugar + Sugar Acid = Glycosaminoglycan
In the previous article, we explored the representative modified sugars, namely amino sugars and sugar acids, in detail. Now, we are finally ready to learn about glycosaminoglycans (GAGs), which are biosynthesized through the repetitive linkage of disaccharide units formed by the combination of one molecule of an amino sugar and one molecule of a sugar acid. If we look at this long name, we see that it ends with "glycan." So what exactly is a glycan?
Glycan
Glycans refer to chains of monosaccharides connected like a string and can also be expressed as "sugar chains." As mentioned in the article on oligosaccharides, terms such as glycans, oligosaccharides, polysaccharides, and carbohydrates are often used interchangeably. Nevertheless, it’s worth revisiting this point lightly in the context of polysaccharides. Both glycans and polysaccharides refer to “compounds composed of many monosaccharides linked by glycosidic bonds” and can be considered synonyms. However, in actual usage, the two terms are employed in slightly different contexts. While polysaccharides refer to independent structures composed solely of monosaccharides, glycans are often used to refer specifically to the carbohydrate portion that is bound to glycoproteins or glycolipids.
While carbohydrates—including monosaccharides, oligosaccharides, and polysaccharides—mainly function as energy sources, energy storage, or structural support, glycans can exist independently or be bound to other biomolecules like proteins and lipids. They play diverse roles such as transmitting signals between cells, modulating immune responses, guiding proper protein folding during synthesis, and protecting proteins from proteolytic enzymes to extend their lifespan.
In the immune response of the complement system, specific glycans on pathogens are recognized by proteins like lectins, which bind to these glycans and activate the complement system via the lectin pathway. During inflammation, for leukocytes to migrate to the site of injury, P-selectin receptors on the endothelial cells must recognize glycans on the leukocyte surface, allowing the leukocytes to adhere, be pulled in, and exit the bloodstream. It is because proteins can recognize distinct, characteristic glycans that signal transmission occurs. Even blood types are determined by the type of glycans present on the surface of red blood cells.
Although personally, I believe glycans perform a broader and more significant range of functions than carbohydrates (polysaccharides), the overlap between the two, along with their differing biological roles, makes direct comparison somewhat ambiguous and perhaps unnecessary.
What is important is that glycans are vital for the proper functioning of biological systems and play major roles in maintaining homeostasis by orchestrating diverse and complex physiological functions. In fact, recent interest in glycans has been growing, as abnormal patterns of glycans on cell surfaces or secreted proteins have been observed in diseases like cancer and autoimmune disorders. Studies have shown that structurally and functionally altered glycans may contribute to cancer progression, metastasis, and immune evasion. This has led to the exploration of glycans as potential biomarkers for disease diagnosis and even the development of glycan-based vaccines and targeted therapies. Furthermore, researchers are working on drug delivery systems that utilize the specific targeting abilities of glycans to deliver therapeutics only to tissues or cells expressing specific glycan receptors.
One reason we can distinguish pathogens from our own cells is that we have unique sugar chains, making glycans an essential part of our biological identity. The broad functions and roles of glycans, which cannot be fully covered here, undoubtedly deserve more detailed exploration in the future.
Extracellular Matrix (ECM)

Connective Tissues
Generally speaking, cells with similar functions group together to form tissues, which in turn organize into organs that carry out specific functions. When these organs work together, they make up an organism—like a human being. The various types of cells in our body can be broadly categorized into four tissue types based on their shape and function: epithelial tissue, muscle tissue, nervous tissue, and connective tissue. Among these, connective tissue plays a key role in mechanical support and shock absorption. To maintain flexibility and resilience, water retention is crucial, which results in lower cell density and a larger proportion of ECM in connective tissue. The high water-retention capability of GAGs explains why they are so abundant in the ECM of connective tissue. For example, skin, which is a connective tissue, contains abundant GAGs like hyaluronic acid that draw in water and maintain elasticity. In bone ECM, chondroitin sulfate binds with calcium to ensure strength and flexibility. In cartilage, chondroitin sulfate and keratan sulfate retain large amounts of water to absorb shocks and prevent joint wear.
Why they’re also called Mucopolysaccharides
GAGs are closely related to mucus, which is why they are also called mucopolysaccharides. Since GAGs were first discovered in mucous tissues, they were initially referred to as mucopolysaccharides. Although the term GAG is now considered more accurate, the older term is still in use. GAGs are highly hydrophilic, attracting and binding water to form viscous, gel-like mucus structures. This gel property gives them viscoelasticity, allowing them to absorb mechanical shocks and protect tissues. GAGs are also abundant in mucous tissues such as the vitreous humor of the eye, synovial fluid, and mucous membranes.
Glycoproteins vs. Proteoglycans
Let’s briefly distinguish between glycoproteins and proteoglycans. Most GAGs are bound to protein peptide chains to form complexes called proteoglycans. While both proteoglycans and glycoproteins are biomolecules formed by the combination of proteins and sugars, they differ in structure and function. In glycoproteins, carbohydrates constitute about 10–20% of the molecule, and these carbohydrate structures play a critical role in protein function, such as acting as cell surface receptors, mediating cell-cell interactions, and modulating hormones and immune responses. Proteoglycans, on the other hand, consist of over 50% carbohydrate content (mainly GAGs) and are key components of the ECM. The difference lies in the carbohydrate-to-protein ratio. All proteoglycans are glycoproteins, but not all glycoproteins are proteoglycans. Thus, proteoglycans can be considered a subcategory within the broader class of glycoproteins.
Now let’s begin exploring the roles of GAGs in connective tissues and the ECM. The representative GAGs that make up connective tissues and the ECM include hyaluronic acid (HA), chondroitin sulfate (CS), dermatan sulfate (DS), heparan sulfate (HS), keratan sulfate (KS), and heparin. All GAGs fundamentally contain amino sugars—either N-acetylglucosamine or N-acetylgalactosamine—and are classified based on whether they undergo sulfation or bind to a protein core to form proteoglycans. Among them, hyaluronic acid is the only one that is neither sulfated nor bound to a protein chain, existing independently. All others are sulfated and attached to protein cores. Sulfated GAGs bind selectively to proteins based on their sulfation patterns, thereby performing specific physiological functions. Let's begin with hyaluronic acid, the most well-known yet structurally simplest of the GAGs.
1. Hyaluronic Acid: N-Acetylglucosamine + Glucuronic Acid
Hyaluronic acid is a polysaccharide in which one amino sugar and one uronic acid form a disaccharide unit, and countless numbers of these disaccharides are repetitively connected. Specifically, the 1st carbon of N-Acetylglucosamine forms a glycosidic bond, that is, a β(1→4) bond, with the 4th carbon of Glucuronic Acid to form a disaccharide. Between these disaccharides, the 1st carbon of Glucuronic Acid forms a glycosidic bond, that is, a β(1→3) bond, with the 3rd carbon of N-Acetylglucosamine.
Unlike other GAGs, which undergo sulfation and further modification in the endoplasmic reticulum and Golgi apparatus, hyaluronic acid has a simple structure without sulfate groups and is directly synthesized at the cell membrane by hyaluronan synthases, immediately secreted into the extracellular matrix. Because it does not undergo complex processing, it is considered the most simple and independent type of GAG.
Functions of Hyaluronic Acid
Despite its simple structure, hyaluronic acid plays a vital role. As a major ECM component, it supports the function of connective tissue. Found in tissues like skin, eyes, and synovium, hyaluronic acid carries a strong negative charge, which enables it to attract and retain water. The carboxyl group (COOH) of glucuronic acid loses a hydrogen ion (H⁺) in the neutral pH of physiological fluids, becoming COO⁻ and thus negatively charged. This polarity allows it to attract polar water molecules.
Its water-retention capability is remarkable: just ¼ teaspoon of hyaluronic acid can hold about 1/5 of a gallon (approximately 5.7 liters) of water—equivalent to 1 gram binding up to 6 liters. [1] This intense moisture retention provides lubrication and shock absorption for tissues.
Hyaluronic acid is closely associated with joints. Joint capsules connecting bones are lined with synovial membranes, which produce synovial fluid. This fluid acts as a lubricant, reducing friction, absorbing external shocks, and delivering nutrients to the avascular cartilage. The key component of this synovial fluid is hyaluronic acid. Just like lubricated machines operate smoothly, joints function better with adequate hyaluronic acid. However, levels of hyaluronic acid decrease with age, causing joints to stiffen and become inflamed, leading to pain.
Along with chondroitin sulfate, which will be discussed next, hyaluronic acid forms large aggrecan aggregates in the extracellular matrix of cartilage. These aggregates support cartilage structure by absorbing shock in combination with collagen. After examining chondroitin sulfate and keratan sulfate, we will revisit the structure of cartilage. In the aggrecan aggregates within cartilage, hyaluronic acid is observed to be very long and chain-like in shape.
Applications of Hyaluronic Acid
Its excellent water-attracting and moisturizing power is utilized in various cosmetic products and procedures to improve skin that has lost elasticity due to wrinkles. It is also used to treat arthritis by injecting it directly into joints to supplement synovial fluid and reduce inflammation. Corticosteroid injections (steroid injections) have the advantage of quickly relieving pain and swelling due to their strong anti-inflammatory effects, but they may damage cartilage with long-term use. Hence, hyaluronic acid injections are gaining attention as an alternative treatment [2].
Initially, hyaluronic acid was extracted from rooster combs, but now it is produced more affordably through bacterial fermentation, making it more accessible in cosmetics. One important point to mention is that hyaluronic acid is a polysaccharide made up of numerous units of glucuronic acid and N-acetylglucosamine. Depending on the length of the chain, it has different molecular weights—classified as high, low, or ultra-low molecular weight—and its absorption and function differ accordingly. Therefore, it's helpful to consider this when selecting cosmetic products.
2. Chondroitin Sulfate
Unlike hyaluronic acid, which exists independently without being bound to a protein core and is directly synthesized by hyaluronic acid synthase on the cell membrane before being secreted outside the cell, the other GAGs discussed in this article are all synthesized by attaching GAG chains to a protein core. This begins with protein core synthesis in the rough ER, followed by chain attachment and sulfation in the Golgi apparatus. The introduction of negatively charged sulfate groups increases hydrophilicity, allowing for better water retention. Chondroitin sulfate is a key component of aggrecan, a major proteoglycan in the extracellular matrix of cartilage. Aggrecan is a cartilage-specific proteoglycan that intertwines with collagen fibers to provide elasticity and shock absorption in the ECM of cartilage.
The name "chondroitin" is derived from the Greek word "chondros," meaning cartilage, indicating that it is mainly found in cartilage tissue. Chondroitin is a disaccharide GAG composed of N-Acetylgalactosamine (GalNAc), an amino sugar, and Glucuronic Acid (GlcA), a uronic acid, which are repetitively bonded. As shown in the diagram, the 1st carbon of GalNAc forms a β(1→4) glycosidic bond with the 4th carbon of GlcA, and the 1st carbon of GlcA forms a β(1→3) glycosidic bond with the 3rd carbon of GalNAc in alternating repetition. The main difference from hyaluronic acid is that chondroitin uses GalNAc instead of GlcNAc as its amino sugar.
Sulfation of Chondroitin
The process of adding sulfate groups (-OSO₃⁻) to specific positions of chondroitin is called sulfation. The addition of negatively charged sulfates enhances water retention in cartilage. Various positions can be sulfated: the 4th and 6th carbon of GalNAc and the 2nd carbon of GlcA. There are various subtypes depending on the sulfation pattern, which slightly affects biological properties. Sulfation patterns selectively regulate binding proteins. For instance, Chondroitin-4-Sulfate enhances cartilage structural stability, while Chondroitin-6-Sulfate is abundant in neural tissue and plays an important role in neuroprotection. In the body, chondroitin predominantly exists in sulfated forms
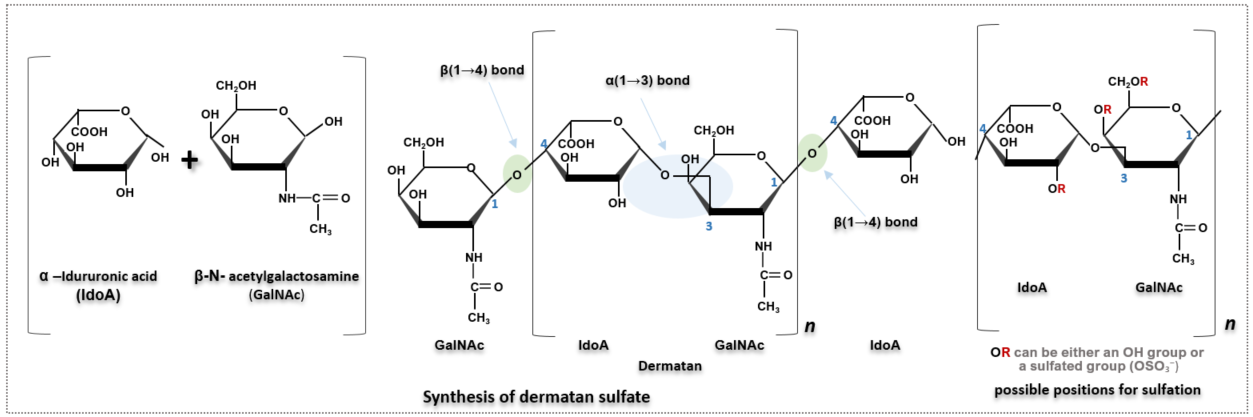
3. Dermatan Sulfate
Unlike chondroitin sulfate, which contains glucuronic acid, dermatan sulfate is composed of iduronic acid (IdoA). IdoA is an epimer of glucuronic acid, differing only in the stereochemistry of the C5 carbon. While the hydroxyl group at C5 of glucuronic acid is positioned downward (axial), in IdoA it is oriented upward (equatorial), allowing free conversion between chair and boat conformations. Although this difference occurs at just one carbon atom, it significantly increases the molecule’s structural flexibility. This structural difference translates to functional variation: while glucuronic acid contributes to the formation of stiff and strong cartilage ECM in chondroitin, IdoA’s flexible structure allows it to bind various proteins and increase elasticity and deformability in tissues such as skin, blood vessels, tendons, and heart valves—tissues where flexibility is more critical.
Dermatan sulfate also has functions similar to heparin, such as strengthening blood vessel walls, regulating blood coagulation, and helping to prevent thrombosis.
Both cellulose, which forms the plant cell wall, and chitin, which constitutes the exoskeletons of animals, have β-linkages that result in linear structures. These linear structures are stabilized by strong hydrogen bonds between the molecules above and below, forming rigid structures. In contrast, IdoA in dermatan sulfate is linked via α-linkages, allowing it to bend and rotate freely. This structural feature contributes to the overall flexibility of dermatan sulfate. Dermatan sulfate also predominantly exists in a sulfated form in vivo, and its sulfation pattern is very similar to that of chondroitin sulfate. The most common form is sulfation at the 4th carbon of GalNAc.
4. Keratan Sulfate
Keratan sulfate is a disaccharide composed of N-Acetylglucosamine (GlcNAc) and Galactose (Gal). Unlike other GAGs, it does not contain uronic acid, which is its most distinctive feature. Sulfation occurs mainly at GlcNAc, and occasionally at Gal. When both molecules are sulfated, it is called di-sulfated keratan sulfate; when only GlcNAc is sulfated, it is called mono-sulfated keratan sulfate. Sulfation rarely occurs at Gal alone. In both molecules, sulfation occurs at the 6th carbon
Keratan sulfate is classified into three types depending on where it is found in the body, with slight differences in function and protein-binding modes:
◾ KS-I: Corneal Type (found in the cornea)
Keratan sulfate was first discovered in the cornea, which also contains the highest concentration of KS in the human body. It is essential for maintaining corneal transparency, and its deficiency can lead to corneal opacity or conditions like macular corneal dystrophy. Sulfation mainly occurs at the 6-O position of GlcNAc, and KS-I connects to proteins via N-linkages to the NH₂ group of asparagine residues.
◾ KS-II: Skeletal/Cartilage Type (found in cartilage and bone)
Along with chondroitin sulfate, keratan sulfate is a major component of cartilage. The massive aggrecan aggregates formed by keratan sulfate, chondroitin sulfate, and hyaluronic acid allow cartilage to absorb shock and support body weight under pressure, preventing wear and tear. Sulfation mainly occurs at GlcNAc 6-O, with occasional 6-O sulfation at Gal as well. KS-II is connected to the protein core via O-linkages to the OH group of serine or threonine residues.
◾ KS-III: Neural Type (found in nervous tissue)
After the cornea, the brain contains the second-highest concentration of keratan sulfate. It is also found in intervertebral discs and the placenta. Sulfation mainly occurs at GlcNAc 6-O, with some Gal 6-O sulfation as well. Heavily sulfated KS-III chains are found in large keratan sulfate proteoglycans in the brain, where they are involved in neural signaling.
One representative proteoglycan is keratan sulfate abakan (Abakan), which is associated with astrocytes and plays roles such as preventing excessive neurite outgrowth, blocking adhesion between neurons, and helping to form boundaries between developing brain regions.[3] Neurites are structural extensions of neurons that enable signal communication and include axons and dendrites.
Another molecule, claustrin, also acts as an anti-adhesive factor, preventing excessive neurite growth and neuron-to-neuron adhesion. These functions may inhibit brain development or neural tissue repair in some contexts, but in others, they help refine and stabilize proper neural circuits by suppressing unnecessary neuronal connections. At least during early brain development, these molecules are thought to be essential in establishing neuron boundaries and supporting healthy brain formation.
SV2 (Synaptic Vesicle Glycoprotein 2)
Another keratan sulfate proteoglycan, SV2, is closely associated with tissues in the heart and the central/peripheral nervous systems (CNS/PNS) that generate electrical signals. Previously, the mechanism of neurotransmitter-mediated signal continuation across the synaptic cleft between neurons was explained, including the role of ion channels and the regulation of ion concentrations.
When an electrical signal reaches the end of a neuron and can go no further, calcium channels at the terminal open, allowing an influx of Ca²⁺ ions into the neuron. This influx triggers the release of pre-formed neurotransmitters enclosed in vesicles into the synaptic cleft. Depending on whether the neurotransmitter is excitatory or inhibitory, it influences which ion channels open on the next neuron, thus affecting whether the next signal is amplified or dampened. Therefore, vesicles that transport neurotransmitters to the synapse are crucial for signal transmission. A key component of these vesicle membranes is synaptic vesicle glycoprotein 2A (SV2A), which contains keratan sulfate.
Generation of Action Potentials in Neurons:
Mice lacking SV2A show abnormal neural signaling and epileptic seizures, suggesting that SV2A is essential for normal nervous system function. [4] Some studies also suggest that SV2A plays a role in calcium storage, thereby influencing calcium concentrations during neurotransmitter release and contributing to the generation of action potentials. Interestingly, this calcium storage capability of keratan sulfate also plays a regulatory role in eggshell formation in birds.
[References]
[1] Hyaluronic Acid
https://my.clevelandclinic.org/health/articles/22915-hyaluronic-acid
[2] Hyaluronic Acid
https://www.ncbi.nlm.nih.gov/books/NBK482440/
[3] Keratan sulfate, a complex glycosaminoglycan with unique functional capability
https://pmc.ncbi.nlm.nih.gov/articles/PMC5993099/#cwy003F1
[4] Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A)
https://pmc.ncbi.nlm.nih.gov/articles/PMC24809/