Beyond monosaccharides and disaccharides, which are composed only of three elements—carbon (C), hydrogen (H), and oxygen (O)—various functional sugars with modified sugar structures emerge at the stage where oligosaccharides and polysaccharides are formed. A monosaccharide can be modified and transformed into a new functional sugar through various reactions in which parts of the monosaccharide structure, such as the aldehyde group (-CHO), hydroxyl group (-OH), or CH₂OH (hydroxymethyl group), are substituted with other elements or oxidized. These modifications expand the functional roles that sugars can perform in the body and enable them to play important roles in cell structure and signal transmission. Before examining their specific functions, let’s first take a detailed look at how these various modified sugars are generated.
I. Amino sugars
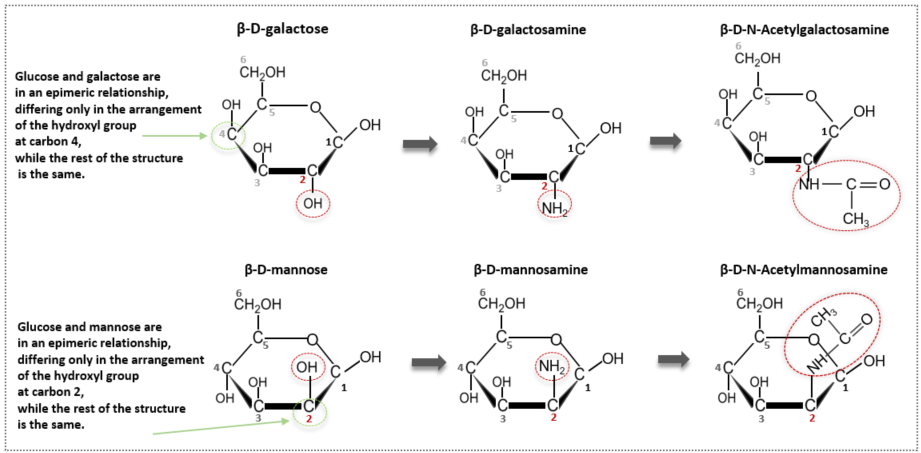
Amino sugars are modified sugars formed by the combination of a sugar and an amino group (-NH₂), which is a key functional group of amino acids that make up proteins. One of the many hydroxyl groups (-OH) present in a monosaccharide is replaced with an NH₂ group. More precisely, the OH group at the 2nd carbon of the monosaccharide is replaced with an NH₂ group. Although more than 60 amino sugars exist, we will focus on three representative amino sugars: glucosamine, galactosamine, and mannosamine. Looking at the names, each is an independent compound formed by substituting an amino group into glucose, so glucose + amine becomes glucosamine. Therefore, galactose becomes galactosamine, and mannose becomes mannosamine. The names are very simple and user-friendly.
Additional acetylation (-NHCOCH₃)
After introducing the amino group, we go one step further and combine the amino group (-NH₂) with acetic acid (CH₃COOH). During this process, a water molecule is removed, and an acetyl group (-COCH₃) remains, resulting in acetylation (-NHCOCH₃). Adding an acetyl group gives the molecule structurally more stable properties and allows it to regulate molecular polarity by balancing hydrophilicity and hydrophobicity. When glucose and galactose undergo these two processes, they are modified and transformed into N-Acetylglucosamine (GlcNAc) and N-Acetylgalactosamine (GalNAc), respectively. The acetylation process of glucose (in its beta form) is illustrated in the diagram below.
Galactose and mannose are also acetylated in the same way to become N-acetyl galactosamine and N-acetyl mannosamine, respectively. As seen in the previous article, glucose and galactose differ only in the configuration at one chiral center (carbon 4), making them epimers. Likewise, glucose and mannose are also epimers at carbon 2. However, galactose and mannose are not epimers of each other because they differ in the configuration at two positions.
Since the names are long, abbreviations are often used. N-Acetylglucosamine is abbreviated as GlcNAc, N-Acetylgalactosamine as GalNAc, and N-Acetylmannosamine as ManNAc. Remembering these abbreviations is very useful later in glycoprotein binding and GAG (glycosaminoglycan) synthesis.
Main component of glycosaminoglycan (GAGs)
Glucosamine (β-D-Glucosamine, C₆H₁₃NO₅) is naturally synthesized in the human body from glucose and glutamine. Amino sugars like glucosamine exert many important functions only after being stabilized through acetylation. For example, GlcNAc is closely related to cartilage health. Cartilage tissue, due to its functional characteristics, has a very rich extracellular matrix, which performs functions such as shock absorption and weight support. This matrix consists of proteoglycans that retain water and absorb impact, collagen that provides structural support, and glycosaminoglycans (GAGs) such as hyaluronic acid and chondroitin sulfate. Here, GAGs deserve special attention. They are important components of the extracellular matrix of all human cells and have a structure in which disaccharides are repeatedly linked. One unit of these disaccharides is an amino sugar like N-Acetylglucosamine (GlcNAc) or N-Acetylgalactosamine (GalNAc). These disaccharides, composed of one amino sugar unit and one sugar acid unit, are repeatedly linked to biosynthesize GAGs such as hyaluronic acid, chondroitin sulfate, keratan sulfate, and heparin. The detailed process will be covered in the next section after discussing sugar acids, another type of modified sugar.
chitin
N-Acetylglucosamine not only becomes a disaccharide unit with sugar acids to form the main components of GAGs but also serves as a major component of various biological structures on its own, such as the exoskeletons of insects, the hard shells of crustaceans like shrimp and crabs, and the cell walls of fungi and molds. Chitin is a polysaccharide where GlcNAc units are linked repeatedly via β(1→4) bonds. It is the second most abundant biopolymer polysaccharide in nature after cellulose. While alpha linkages mainly appear in energy storage, beta linkages are formed in structural roles like those of cellulose and chitin. It is fascinating that a sugar, often associated with sweetness, becomes the hard and rigid component of shells and exoskeletons.
Similarities Between Chitin and Cellulose
The glycosidic bonds in chitin are very similar to those in cellulose. While cellulose is composed of β-anomeric forms of glucose, chitin consists of β-anomeric forms of N-Acetylglucosamine. Except for the fact that chitin is harder due to the presence of the acetyl group, the two are quite similar. Both form β(1→4) glycosidic bonds. In these bonds, the OH groups at carbon 1 and carbon 4 are oriented differently, so the units flip-flop alternately to bond naturally. This results in a straight, linear chain with no branches. The top and bottom of these long linear chains are further connected through hydrogen bonding, forming strong and rigid structures like plant cell walls or animal exoskeletons. The human body does not have enzymes that can break down these β-glycosidic bonds, which is why dietary fibers cannot be digested. Both chitin and cellulose are also homopolysaccharides, composed of only one type of monosaccharide unit.
II. Sugar acid
A monosaccharide with a carbonyl group located at the end of its structure in the form of an aldehyde is called an aldose. When aldoses such as glucose or galactose are oxidized, they become sugar acids. Depending on the site of oxidation—whether it is the aldehyde group on carbon 1 or the terminal hydroxymethyl group (-CH₂OH) on carbon 6—they are classified as aldonic acids and uronic acids, respectively. There is also aldaric acid, where both carbon 1 and carbon 6 are oxidized, but it is not considered significant since its essential metabolic function in the body is not well known. Aldonic acids may appear as intermediate metabolites in the body, but they are not known to play a central role in major metabolic pathways. Therefore, the most noteworthy sugar acids that will be discussed here are the uronic acids, which perform essential and central functions in the body.
A uronic acid is a sugar in which the sixth carbon of a monosaccharide has been oxidized into a carboxylic acid group (-COOH). Depending on which monosaccharide has been oxidized, various uronic acids are formed—such as glucuronic acid (GlcA), galacturonic acid (GalA), mannuronic acid (ManA), and iduronic acid (IdoA). These play crucial roles in biological functions and are not obtained directly from food but are synthesized through metabolic processes within the body. The two most biologically significant uronic acids are glucuronic acid (GlcA), which is derived from glucose, and iduronic acid (IdoA), an epimer of glucuronic acid differing only in the stereochemistry at carbon 5. Unlike glucuronic acid, iduronic acid is characterized by its structural flexibility due to free rotation between carbon 1 and carbon 5.
Liver Detoxification: Phase I and Phase II
To understand the importance of glucuronic acid (GlcA) in the human body, we can examine the liver’s detoxification process. The liver is known to detoxify drugs, harmful substances, and toxins that have entered the body and fulfilled their roles. While the term "detoxification" might be misunderstood to mean neutralizing or rendering a toxin harmless, what the liver actually does is transform these harmful substances into forms that can be easily excreted from the body.
More specifically, most drugs and toxic substances are hydrophobic, non-polar, and lipophilic—meaning they repel water and easily penetrate cell membranes made of lipid bilayers, allowing them to accumulate inside cells. The human body has two main ways to excrete unwanted substances: through the kidneys into the urine or through bile into the feces. Small, hydrophilic molecules are filtered and excreted in the urine without being reabsorbed in the kidneys. Larger toxic molecules are excreted into the intestines via bile and then removed through the feces.
Thus, liver detoxification transforms hydrophobic, lipophilic, non-polar toxins into hydrophilic, water-soluble, polar substances. This detoxification process involves various chemical modifications and occurs in two phases: Phase I, which involves oxidation, reduction, and hydrolysis reactions primarily mediated by the cytochrome P450 enzyme family (CYP450); and Phase II, which follows if the intermediate products of Phase I still possess toxicity. Phase II involves conjugating more strongly polar molecules to the intermediate to increase solubility and polarity.
The key objective of liver detoxification is rapid elimination. In Phase II, various groups such as acetyl, sulfur, glutathione (GSH), and glucuronic acid are added. Among these, glucuronidation—the addition of glucuronic acid—is the most important and common mechanism.
Glucuronic Acid in Bilirubin Excretion
Glucuronic acid (GlcA) is also essential for the excretion of bilirubin, in addition to metabolized drugs and toxins. When red blood cells reach the end of their lifespan, they are broken down. The iron (Fe²⁺) and globin proteins are recycled, but heme, the oxygen-carrying core, is not. This heme is broken down into bilirubin, which is then processed in the liver. When glucuronic acid is attached to bilirubin, it becomes more polar and water-soluble and is excreted through bile.
If this process fails and bilirubin is not properly excreted, it can accumulate, leading to jaundice—a yellowing of the skin and eyes. Once excreted in bile and broken down by gut microbiota, bilirubin is converted into urobilinogen, which is further metabolized into stercobilin and urobilin, giving feces its brown color and urine its yellow color, respectively. Interestingly, glucuronic acid was first discovered in urine, which is why its name includes the “-uronic” suffix, derived from urine.
Glucuronic Acid in Hormone Excretion
Glucuronic acid is also responsible for hormone excretion. Lipophilic steroid hormones such as cortisol, estrogen, and testosterone, as well as thyroid hormones, are designed to act only for a limited time in the body. If they linger too long or accumulate excessively, they may produce undesired physiological effects and disrupt hormonal balance.
For example, while an increase in cortisol during stress is a normal response, prolonged elevation can lead to immune suppression and other side effects. Therefore, after performing their roles, these hormones must be promptly eliminated. In the liver, glucuronic acid conjugates with these hormones, making them water-soluble so they can be excreted through urine or bile.
Biosynthetic Pathway of Glucuronic Acid in the Body
When glucose enters human cells through specialized transporters (GLUT), the cell immediately adds a negatively charged phosphate group (PO₄³⁻) to trap it inside. This phosphorylation step ensures that the now charged and polar glucose molecule cannot pass back out through GLUT. The resulting compound is glucose-6-phosphate (G6P), which serves as the starting point for all glucose oxidation and metabolic activities.
From here, G6P can follow multiple metabolic routes. It may proceed through glycolysis to produce pyruvate for ATP generation; it may be converted into glucose-1-phosphate (G1P) to form glycogen or glucuronic acid; or it may enter the pentose phosphate pathway (PPP) to produce NADPH—an essential reducing agent and antioxidant—or to synthesize ribose for DNA and RNA production. The path taken depends on the cell’s condition and needs—whether energy is required, whether excess ATP should be stored, whether oxidative stress requires enhanced antioxidant defense, whether nucleic acids need to be synthesized, or whether detoxification requires excretion of harmful substances. These metabolic decisions are precisely regulated.
The metabolic pathways derived from G6P are even more diverse than described here. Among them, let's examine the pathway by which glucose is biosynthesized into glucuronic acid, giving us insight into uronic acid synthesis.
Glucuronic acid is not obtained through diet but synthesized within the body, primarily existing in the form of UDP-glucuronic acid. This activated form is essential for detoxification and for synthesizing substances such as hyaluronic acid and proteoglycans. The uridine diphosphate (UDP) component increases the reactivity and recognizability of the molecule by enzymes, facilitating efficient biochemical reactions.
Once synthesized via the steps illustrated above, UDP-glucuronic acid is utilized in various metabolic pathways. Interestingly, although humans lack the necessary enzyme to complete this process, one of the potential uses of UDP-glucuronic acid in other species is the biosynthesis of vitamin C (ascorbic acid).
Having reviewed amino sugars and sugar acids, we are now ready to explore how these two types of building blocks combine in equal parts to form disaccharides, which then polymerize repeatedly to form the polysaccharides known as GAGs (glycosaminoglycans). These vital macromolecules, found in the extracellular matrices of the skin, joints, retina, blood vessels, and other tissues, play key roles in water retention, elasticity, shock absorption, cellular signaling, anticoagulation, and immune modulation. The discussion of GAGs will continue in the next article.