Lester Packer, a professor of biochemistry at the University of California, Berkeley, has dedicated his career to antioxidant research and has written 800 scientific papers and more than 100 books related to antioxidants and health, earning him the nickname "Antioxidant Doctor." [1] In “The Antioxidant Miracle,” his first book written for non-scientific audiences, he argued that antioxidants do not function individually but rather collaborate within networks, where antioxidants regenerate each other. The network as a whole is a “miracle” that forms a protective barrier against diseases and slows down the aging process.
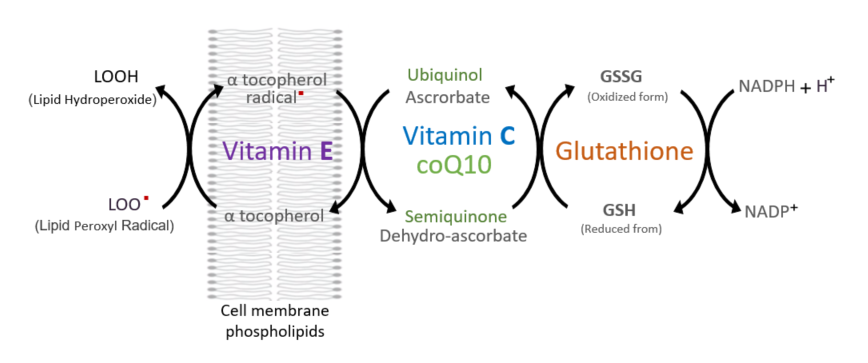
As we know, antioxidants play a crucial role in disarming radicals that have become unstable by losing an electron. By willingly donating their electrons, antioxidants prevent these radicals from causing harm. However, in this noble act of neutralizing radicals, antioxidants themselves become mildly radicalized due to the loss of an electron. While these newly formed radicals are significantly less harmful than the ones they neutralized, they are still radicals. Therefore, there's a need for a system to regenerate these spent antioxidants, restoring them to their original state. This is where the concept of the antioxidant regeneration network comes into play. In this network, Various antioxidants work together to regenerate each other, forming a cycle of antioxidant activity. For example, when vitamin C is oxidized, glutathione reduces it; when glutathione is oxidized, alpha lipoic acid reduces it; when alpha lipoic acid is oxidized, coenzyme Q10 reduces it; and when CoQ10 is oxidized, vitamin E reduces it. Finally, oxidized vitamin E is regenerated by vitamin C, completing the cycle of antioxidant regeneration.
Professor Packer emphasized the importance of alpha lipoic acid as a central antioxidant in the network due to its unique properties. Unlike other antioxidants, alpha lipoic acid not only regenerates other antioxidants within the network but also has the ability to regenerate itself. Additionally, while vitamin C and glutathione are water-soluble and primarily located in the cytoplasm, and vitamin E and CoQ10 are fat-soluble and found in cell membranes, alpha lipoic acid possesses both water-soluble and fat-soluble properties. This dual solubility enables alpha lipoic acid to have a broader range of activity, making it particularly useful in combating oxidative stress.
To begin with, let’s briefly look at each antioxidant before dealing with the network system.
Glutathione
Glutathione, composed of the synthesis of three amino acids glutamate, cysteine, and glycine, plays a crucial role in the body's oxidative-reduction reactions. The sulfhydryl groups (-SH groups, often referred to as thiol groups) made up of the sulfur and hydrogen of cysteine are an important site where redox reactions occur. It is almost absent in the blood and mostly exists within cells and operates effectively in aqueous environments. Glutathione exists in two forms: the reduced form (GSH), which is the normal state, and the oxidized form (GSSG). In healthy cells and tissues, GSH typically constitutes over 90% of the total glutathione pool. The ratio between the oxidized and reduced forms, known as the GSH:GSSG ratio, serves as a measure of oxidative stress. Under normal conditions, this ratio is approximately 100:1. However, in situations of severe oxidative stress, such as exposure to high levels of free radicals, this ratio can decrease significantly, potentially reaching levels as low as 1:1. This change in the GSH:GSSG ratio reflects the extent of oxidative damage occurring within cells and tissues.
Four major functions of glutathione
1. Antioxidant action: As seen in the antioxidant enzyme action discussed in the previous articles, it is used in the GPx antioxidant catalytic enzyme. It is recognized as the most powerful antioxidant and has the advantage of being almost non-toxic even after oxidation. In addition, Glutathione exhibits antioxidant effects by actively participating in the reduction and regeneration of oxidized forms of other antioxidants such as vitamin C and vitamin E, thereby enabling them to restore their antioxidant activity.
2. Glutathione serves as a powerful detoxifying agent in the body by actively eliminating various harmful substances from the body, including toxic chemicals like formaldehyde and chlorine gas, heavy metals such as lead, cadmium, and mercury, drug metabolites, and environmental pollutants. It achieves detoxification through a process known as chelation, wherein glutathione binds to these compounds and facilitates their safe excretion from the body via bile and feces, primarily facilitated by the enzyme glutathione-S-transferase.[2]
3. Immunity-enhancing effect: Glutathione enhances immune function by promoting the maturation and activation of T cells.
4. Increases fat-burning effect: Glutathione regulates the oxidation of fatty acids in mitochondria, thereby enhancing fat-burning and increasing energy production.
With its function of promoting cell health and helping detoxification, it is used as a clinically beneficial component, even if not as a cure, for neurodegenerative diseases, lung diseases, immune diseases, cardiovascular diseases, liver diseases, etc. Since the brain, whose oxygen consumption is much higher than that of other organs and therefore is much more vulnerable to attack by free radicals, glutathione's antioxidant properties can be most beneficial in maintaining brain health.
Alpha Lipoic Acid (ALA)
ALA is an organosulfur compound containing 8 carbon atoms(C8H14O2S2). It acts as an important Cofactor in energy metabolism, participating in the removal of one carbon atom and its subsequent combination with coenzyme A(CoA). This ALA’s function happens within the tricarboxylic acid(TCA) cycle in two enzyme complexes: Pyruvate Dehydrogenase Complex, which converts Pyruvate into acetyl coA, and α-Ketoglutarate dehydrogenase complex, which converts α-Ketoglutaric acid into succinyl-coA.
As an antioxidant, Alpha-Lipoic Acid (ALA) plays a pivotal role within the antioxidant network due to its ability to regenerate not only other antioxidants within the network but also itself. In addition, unlike other antioxidants, it has both water- and fat-soluble properties, allowing it to act across a broader spectrum of cells. So it is often called a universal antioxidant. ALA is an organosulfur compound synthesized in small quantities daily within mitochondria and found in all cells of the human body.
Oxidative stress induced by hyperglycemia can lead to programmed cell death of neurons, triggering the development of diabetic neuropathy. When ALA is administered orally, it has been reported to improve symptoms of neuropathy. In experimental diabetic neuropathy, ALA has been shown to improve motor nerve conduction velocity and protect peripheral nerves from ischemia.[3]
What's also notable is that when ALA is utilized for treatment, it enhances the levels of reduced glutathione (GSH) in vivo. Research conducted by Professor Packer has indicated that ALA can elevate glutathione levels by as much as 30%.[4]
ALA is also well known for its remarkable anti-inflammatory properties, and in a meta-analysis involving patients with metabolic syndrome and related conditions, ALA supplementation showed a reduction in markers of inflammation such as interleukin 6 (IL-6), tumor necrosis factor-alpha(TNF-α), and C-reactive protein(CRP: a substance produced by the liver in response to inflammation). These findings suggest that ALA diminishes inflammation by decreasing CRP levels and preventing the excessive activation of the nuclear factor κB(NF-κB) signaling pathway, which is responsible for transcribing cytokine production.[5]
Vitamin E
It exists in eight forms in the human body, but alpha-tocopherol is the most common form and has the highest bioavailability. As a fat-soluble antioxidant, its most important function is to play a major role in preventing fatty oxidation of cell membrane phospholipids and other fatty acids. It is a primary defense antioxidant against lipid peroxidation, terminating the chain reaction caused by free radicals in this process. Through this mechanism, it protects lipids in the blood and tissues, thus preventing the oxidation of low-density lipoprotein (LDL) and improving cardiovascular health. This antioxidant plays a crucial role in preventing the formation of cholesterol oxidation products, which can lead to heart attacks and strokes. In previous articles, we explored how ongoing lipid peroxidation can have detrimental effects on lipid-containing cells and contribute to the formation of harmful aldehydes.
Vitamin E has been shown to improve the formation of adherens junctions (known as immune synapses) between T lymphocytes and antigen-presenting cells (APC), which in turn trigger T cell activation and proliferation, thereby influencing immunity. [7]
Vitamin E has a constant reaction rate against various radicals and acts very quickly, meaning that even a very small amount can effectively fight against the creation of a huge amount of peroxide radicals in the body.
For reference, in 2008, the medical community was thrown into confusion when a research team at Copenhagen University Hospital announced the results of a study showing that taking antioxidant vitamins such as vitamins A, E, and beta-carotene increased the mortality rate by over 5% on average. This report, also known as the 'Copenhagen Shock', has been the subject of much debate since then, but definitive conclusions still remain elusive. The authors, in an update to their report, suggested that antioxidant supplements should be considered as pharmaceuticals and undergo rigorous evaluation before being marketed.[6] Fortunately, fat-soluble vitamins tend to stay in the human body for a certain period of time, and considering this, some argues that obtaining them through dietary sources may be sufficient without the need for supplementation. The recommended daily allowance (RDA) for adults is 15 mg, and it is found in large quantities in olive oil, sunflower seeds, peanuts, almonds, etc. [8]
Lipid peroxidation, vitamin E, and vitamin C
Vitamin E is recognized for its paramount role as an antioxidant, particularly as a fat-soluble vitamin, where it plays a unique and crucial role in lipid-related antioxidant activities. Let's take a closer look at its mechanism of action.
Human cells are enclosed by membranes, which serve as a protective barrier, selectively allowing substances to pass through. These membranes not only distinguish between what should and shouldn't permeate but also play a pivotal role in maintaining the integrity of cells and their internal compartments. This includes the cell membrane that separates the cell's interior from its surroundings, membranes surrounding internal organelles and the nucleus, and membranes forming tissues such as mucous membranes and serous membranes. Even red blood cells are surrounded by membranes.These biological membranes are composed of phospholipids, which consist of two fatty acids and one phosphate group. The structure of phospholipids forms a bilayer, with the hydrophobic (water-repelling) tails, made of fatty acids, facing inward (the green part in the image), and the hydrophilic (water-friendly) heads, made of phosphate groups, facing outward towards the water (the pink part).
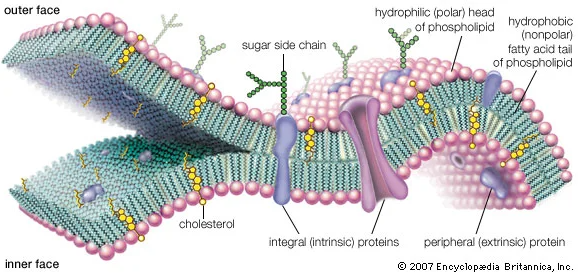
When cell membranes, specifically the unsaturated fatty acids in the phospholipids' tails, face radical attacks, a dangerous chain reaction called lipid peroxidation is triggered. This reaction can cause significant damage to the cell membrane. To prevent this, vitamin E (primarily α-tocopherol) acts as a lipid-soluble antioxidant, donating its electrons to lipid radicals and becoming oxidized into the vitamin E radical (α-tocopheryl radical) itself. To ensure the continuous antioxidant activity of vitamin E, vitamin C is essential. Vitamin C can regenerate oxidized vitamin E back to its original state. The phenolic group of vitamin E's tocopherol, located near the water-friendly part of the cell membrane, allows the hydrophilic vitamin C to easily access and regenerate the tocopherol from its radical form back to its active state. Research has shown that when cell membranes under radical attack are treated with both vitamins C and E, the amount of vitamin E remains unchanged until all vitamin C is depleted, highlighting a synergistic relationship between these vitamins. Since the phenol group of Vitame E is located near the water-friendly part of the cell membrane, hydrophilic vitamin C can easily access the antioxidant activity site of this tocopherol and regenerate back to its active state. [9] Research has shown that when cell membranes under radical attack are treated with both vitamins C and E, the amount of vitamin E remains unchanged until all vitamin C is depleted, highlighting a synergistic relationship between these two vitamins. [10]
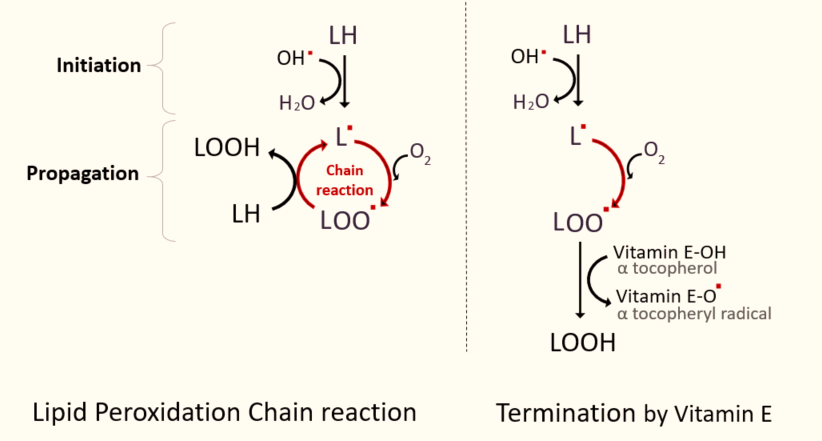
Lipid peroxidation is a chain reaction that can cause significant damage to cell membranes. This process can be simplified into three main stages: initiation, propagation, and termination. During the propagation stage, a lipid radical (L˚) reacts with oxygen to form a lipid peroxyl radical (LOO˚), which then attacks another lipid (LH) to propagate the chain reaction. Vitamin E terminates this propagation stage by donating an electron, preventing further damage. The diagram simplifies the process. (PowerPoint is not easy to work with for people with fat fingers...)
Vitamin C (A scorbic acid)
Vitamin C, a water-soluble component, acts both inside and outside of cells to neutralize radicals. It is an indispensable nutrient that participates in numerous biochemical processes. It is necessary when converting dopamine to norepinephrine in catecholamine biosynthesis in the brain and adrenal glands, and is involved in improving components of the immune system. It is also essential for the synthesis of collagen, since procollagen, the collagen precursor, is converted to collagen only when the proline residue is oxidized to hydroxyproline through vitamin C. However, during evolution, humans and some higher primates lost the ability to produce vitamin C internally due to a genetic mutation that inactivated the gene responsible for expressing the necessary enzyme to synthesize vitamin C, making it necessary to intake from external sources.
Vitamin C, known for its significant role as an antioxidant, is particularly effective at scavenging singlet oxygen(1O2), a type of ROS, at a rate approximately 100 times faster than glutathione. This capability is especially crucial in the cell nucleus and near the genomic DNA, where ROS specialized removal enzymes like SOD(Superoxide Dismutase) are absent.
Redox reaction of vitamin C
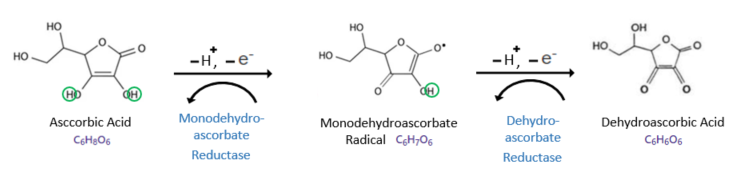
Vitamin C donates only one electron at a time to the radical. Typically, it undergoes oxidation in two stages: first, it donates one proton and one electron to the radical, becoming partially oxidised to Monodehydroascorbate radical (also known as Ascorbate radical or Semidehydroascorbic acid), and then, repeats the same process again to get completely oxidized to Dehydroascorbic acid. During each step, a reductase is required to revert to the previous stage(See the picture below). For reducing dehydroascorbic acid back to vitamin C, glutathione (GSH) is required.
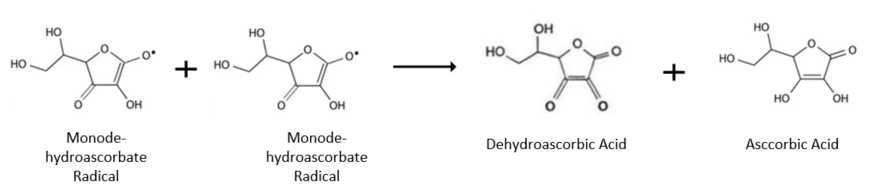
Under physiological conditions, the Monodehydroascorbate radical is typically reduced back to vitamin C by the reductase enzyme. However, in the absence of this enzyme, two Monodehydroascorbate radicals can react with each other spontaneously, undergoing an unaided disproportionation reaction. This results in the formation of one Ascorbic Acid and one Dehydroascorbic Acid. Both of these molecules can be reduced by the same reduction pathway as shown in the above picture.
Vitamin C is mostly absorbed from the intestines through the sodium-dependent vitamin C transporter (SVCT). However, when it enters the cells, it must enter in the oxidized form of dehydroascorbic acid through Glut1, a glucose transporter. Once transported inside the cells, it is then reduced back to ascorbic acid and carries out the activities to protect mitochondria from oxidative stress. Interestingly, the chemical formulas of vitamin C(C₆H₈O₆) and glucose(C₆H₁₂O₆) are very similar.
Coenzyme Q10
CoQ10, which is fat-soluble, goes by various names. Coenzyme refers to a cofactor that helps the catalytic activity of an enzyme, and Q refers to Quinone, which is a general term for compounds in which two hydrogens are replaced with two oxygens in the benzene ring of an aromatic compound. The number 10 is given because the subchemical unit called isoprene is repeated 10 times in the tail. It is also called ubiquinone, after Ubiquitous, to indicate that it exists everywhere in all cells. Its chemical structure is similar to vitamin K and it fits the general definition of a vitamin, so it is sometimes called vitamin Q, but it cannot be considered a vitamin because it is synthesized within the human body.
Due to its crucial role in the electron transport chain for ATP energy synthesis, CoQ10 is found in high concentrations in energy-demanding organs such as the heart, liver, and kidneys. Unlike other coenzymes like NAD or FAD, which transfer two electrons simultaneously, CoQ10 possesses the unique ability to transfer one electron at a time. This characteristic makes it indispensable for the electron transport chain's functioning.
3 Forms of CoQ10
The oxidized form of ubiquinone, CoQ10, undergoes partial reduction to semiquinone by receiving one electron and a proton, and then by repeating the same process, it becomes completely reduced to ubiquinol.
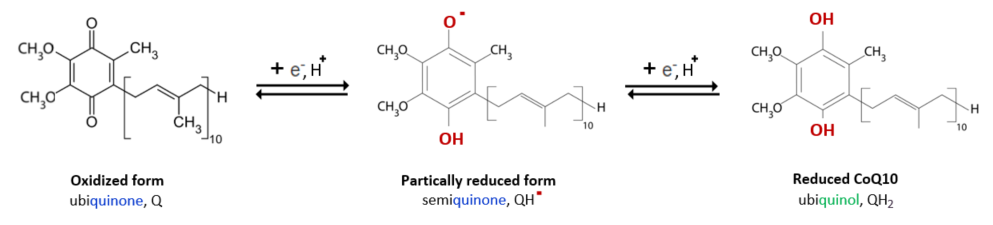
Let's look at the antioxidant activities of CoQ10. First, ubiquinol(CoQH2), a fat-friendly antioxidant, removes radicals by donating electrons to lipid peroxy radicals(LOO˚) during the lipid peroxidation process like vitamin E.
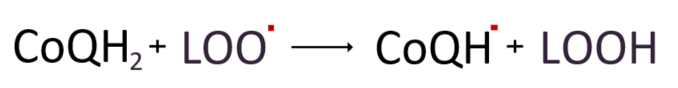
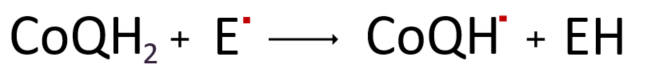
Additionally, it can regenerate oxidized vitamin E˚ through its antioxidant activity.
Semiquinone (CoQH˚), which has been oxidized by donating electrons in the antioxidant process described above, accepts electrons transferred in the electron transport chain(ETC), which is the ATP energy metabolism process, and is regenerated to ubiquinol to initiate antioxidant activity
Supplements
As we are all aware, the rate of biosynthesis within the body significantly decreases with age, and especially for the patients who continuously take Statins additional supplementation is recommended.[11] The reason behind this is that Statin drugs, prescribed for patients with hyperlipidemia, work by inhibiting the HMG-CoA reductase enzyme, thereby reducing the production of cholesterol. However, a side effect of this mechanism is that this inhibition can also reduce the amount of coQ10 produced through the same pathway
The interaction between the antioxidants examined above is graphically represented as follows.
[Reference materials]
[1] LESTER PACKER, PH.D.
https://www.nuskin.com/content/dam/nse/pdf/Bios/lesterpacker.pd
[2] The role of glutathione in detoxication
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1569131/pdf/envhper00455-0063.pdf
[3] Alpha-Lipoic Acid and Diabetic Neuropathy
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2836194/
[4] Review: The Antioxidant Network
https://www.lifeextension.com/magazine/1999/8/report5
[5] The effects of alpha-lipoic acid supplementation on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5989440/
[6] Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8407395/
[7] Vitamin E
https://lpi.oregonstate.edu/mic/vitamins/vitamin-E
[8] Vitamin E
https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
[9] An Overview of the Characteristics and Function of Vitamin C in Various Tissues: Relying on its Antioxidant Function
https://brieflands.com/articles/zjrms-4037
[10] Lipid antioxidants: how they may act in biological systems.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2149475/
[11] Coenzyme Q10: Regulators of Mitochondria and beyond
https://www.intechopen.com/chapters/71878



