The type of hybridization determines the formation of sigma (σ) and pi (π) bonds.
In the previous article, we examined the hybridization steps for the three main types of carbon hybridization. In this article, we aim to explore the types of covalent bonds that result from different hybridization types, since single, double, and triple bonds are logically closely related to the type of hybridization.
When carbon forms covalent bonds, it prepares to generate hybrid orbitals by mixing its s orbital and p orbitals in the second(L) shell, depending on how many bonding partners it will have. After equalizing the energy levels of the two orbitals by promoting an electron, carbon can create four sp³ hybrid orbitals through the process of "sp³ hybridization" when bonding with four atoms. When bonding with three atoms, it uses "sp² hybridization" to form three sp² hybrid orbitals, and when bonding with two atoms, it employs "sp hybridization" to create two sp hybrid orbitals.
This various hybridization by carbon is crucial for forming stronger and more stable sigma bonds. The extent to which orbitals overlap directly indicates the strength of the covalent bond that can be formed. Orbitals represent the probability density of electrons, and significant overlap between orbitals of two atoms implies a higher electron density, leading to stronger bonding. Hybridization enhances the efficiency of overlap and optimizes bond angles, minimizing repulsion between atoms, making it a highly effective process.
Sigma (σ) bonds and pi (π) bonds
Sigma bonds can occur between various types of orbitals. As shown in the diagram below, hybridized orbitals are also an example of sigma bonding. A sigma bond is characterized by electron density that is symmetrically distributed around the axis connecting the nuclei of the two bonded atoms. When we commonly say that "two atoms form a single covalent bond," we are referring to a sigma bond.
In other words, the formation of a sigma bond results in a single bond. Let’s consider hydrocarbons, which are the simplest and most fundamental forms of organic compounds, serving as the backbone to which various functional groups or atoms can be added to form countless organic compounds.
As the name suggests, hydrocarbons are compounds consisting solely of carbon and hydrogen. Depending on how the carbon atoms are bonded within their structure, hydrocarbons can be classified as follows: if they are only connected by single bonds, they are called alkanes; if there is at least one double bond, they are termed alkenes; and if there is a triple bond present, they are classified as alkynes. Generally, in hydrocarbon compounds where carbon is the central atom surrounded by hydrogen atoms, carbon forms single bonds with either hydrogen or other carbon atoms. The most basic form of alkanes is methane, which consists of a single carbon atom at its center. Notably, all alkanes are named using Greek prefixes corresponding to the number of carbon atoms, followed by the suffix “-ane” to indicate they are alkanes. This results in names like Methane, Ethane, Propane, Butane, Pentane, Hexane, Heptane, Octane, Nonane, and Decane.
Returning to sigma bonds, remember that the carbon atoms in alkanes are all connected by single bonds and form four bonds with their surroundings. Therefore, it can be said that alkanes all form four sigma bonds, which is why methane (CH₄), the simplest form of an alkane with one carbon bonded to four hydrogens, frequently serves as an example to explain sp³ hybridization.
Then, what about alkenes and alkynes, which have double bonds? Double and triple bonds fundamentally contain at least one single bond. There cannot be a pi bond without a sigma bond. This means that a double bond consists of one single bond (sigma bond) and one pi bond, while a triple bond consists of one sigma bond and two pi bonds. Unlike sigma bonds, which can form from various orbitals, pi bonds always occur between p orbitals.
Typically, hybridization involves the mixing of s and p orbitals to equalize energy levels, and the remaining unhybridized p orbitals form the pi bonds with each other. However, there are cases where two p orbitals can form a pi bond without hybridization, such as in the nitrogen molecule (N₂) where two nitrogen atoms bond. The key point is that pi bonds are generated from p orbitals. In Greek, p is represented as π. Honestly, I struggled to understand double and triple bonds. No matter how much I thought about it, I couldn't grasp how two atoms could form three covalent bonds. I blamed my limited imagination. How could orbitals overlap three times to form covalent bonds? I was trying to understand it by assuming three identical single bonds. It wasn't until I understood pi bonds that the pieces started to fall into place.
As shown in the diagram, a pi bond occurs when the orbitals of the bonding atoms overlap laterally around the axis that connects their nuclei. This means that electron density does not exist along the axis between the nuclei; instead, it is distributed vertically above or below, or to the right or left of the axis. This represents a completely different type of bonding compared to sigma bonds.
A pi bond is always formed in addition to a sigma bond that has already been created. Now that we have briefly explored sigma and pi bonds, as well as single, double, and triple bonds, let’s delve deeper into the connection between these bonds and hybridization.
The relationship between Alkane hydrocarbons and hybridization types
Hydrocarbons composed of carbon and hydrogen can be classified into alkanes (which have only single bonds), alkenes (which contain double bonds), and alkynes (which contain triple bonds). Among these, alkanes make up the largest proportion found in nature. The carbon atoms in alkanes form a total of four covalent bonds with either carbon or hydrogen, and these bonds are all single bonds, meaning they all form sigma (σ) bonds.
Alkane compounds can have various structures, such as linear chains (like methane, CH₄), branched structures, or cyclic forms (like cyclohexane, C₆H₁₂). Regardless of the structure, any arrangement where carbon atoms are connected solely by single bonds to other carbon or hydrogen atoms is considered an alkane. Therefore, the sp³ hybridization that occurs to enable carbon to form four single bonds is inextricably linked to the nature of alkanes.
In contrast, alkenes, which contain double bonds, are associated with sp² hybridization, while alkynes, which contain triple bonds, are related to sp hybridization. The newly formed orbitals resulting from hybridization are involved in creating the sigma bonds, while the remaining p orbitals that did not participate in hybridization form the pi bonds. Let’s examine this in more detail.
Alkanes and sp³ hybridization
First, let’s explore how the simplest alkanes, methane and ethane, are demonstrated using various representations. Note that the four hybrid orbitals each form a single (sigma) bond with the four hydrogen atoms.
Alkenes and sp² hybridization
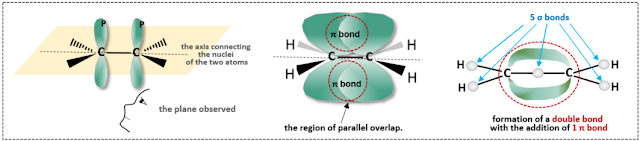
Alkenes are hydrocarbon compounds that contain one or more double bonds. Let’s examine the relationship between sp² hybridization, double bonds, and pi bonds using ethylene as an example. Ethylene has a structure consisting of two carbon atoms bonded to four hydrogen atoms. It is well known as a fruit ripening hormone that accelerates the ripening process.
Looking at its structure, the two carbon atoms each undergo hybridization to produce three sp² orbitals and one remaining unhybridized p orbital. Therefore, a total of six sp² orbitals and two p orbitals are involved in forming total 5 single bonds (sigma bonds) and 1 pi bond.
Looking at the structure of carbon atoms undergoing sp² hybridization, the three sp² orbitals are arranged in a planar triangular geometry, maintaining an angle of 120॰ between them. This structure can be explained by the Valence Shell Electron Pair Repulsion (VSEPR) theory, which states that since all electrons carry a negative charge, there will be a repulsive force between electron pairs of the same charge. To minimize this repulsive force and maintain a lower energy level for stability, the electron pairs bonded to the central atom try to be as far apart as possible. This characteristic allows us to predict the geometric structure of the molecule. In the case of sp² hybridization, the presence of three electron pairs around the central atom leads to an optimal structure where these pairs maintain a 120॰ angle due to electron pair repulsion. The unhybridized p orbitals are positioned perpendicularly to this plane.
Considering the molecular structure of ethylene, we can illustrate how the two carbon atoms are arranged for bonding, as shown in the figure above.
The hybrid orbitals of the two carbon atoms are all situated in a single plane, which is highlighted in yellow. To add a sense of perspective, the side closer to the observer is represented with a wedge shape, while the side farther from the observer is indicated with a thick dashed line, creating a three-dimensional representation. The two carbon atoms form a total of five single (sigma) bonds by overlapping their orbitals with four hydrogen atoms and one between carbon atoms. Additionally, the remaining two p orbitals align parallel to each other and overlap, facilitating the formation of the bond. In reality, the overlap of the p orbitals occurs over a relatively large area, allowing electrons to be shared in that overlapping space, thus forming a pi (π) bond.
While sigma bonds overlap along the axis connecting the nuclei of the two bonding partners, pi bonds do not pass through the nuclei; instead, they overlap parallel to the nuclei, either above or below, and in the case of triple bonds, also to the left and right. Sigma bonds form very strong connections, whereas pi bonds are relatively weaker and more easily broken. Atoms involved in sigma bonding can rotate freely, but when additional pi bonds are formed, this rotation becomes restricted. In other words, atoms involved in double or triple bonds have limited freedom of rotation.
Alkynes and sp Hybridization
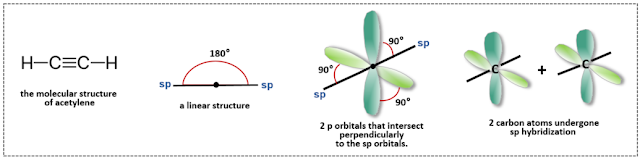
Let’s use acetylene (C₂H₂) as an example to illustrate how triple bonds are formed and to show the simplest form of sp hybridization. Acetylene is a compound consisting of two carbon atoms and two hydrogen atoms, characterized by having one triple bond, making it an alkyne hydrocarbon. According to the Valence Shell Electron Pair Repulsion (VSEPR) theory, sp³ hybridization exhibits a tetrahedral geometry with bond angles of 109.5°, while sp² hybridization has a planar triangular geometry with bond angles of 120°. In contrast, sp hybridization is predicted to result in a linear structure with bond angles of 180°, as the electron pairs are positioned as far apart as possible.
The two sp hybrid orbitals, pointing in opposite directions, are positioned perpendicularly to the two p orbitals that are not involved in hybridization. It seems quite reasonable that these two p orbitals would overlap with the orbitals of another atom to form pi bonds. As a result, with the formation of two pi bonds, a triple bond is ultimately formed.
Double bonds and triple bonds
As previously mentioned, both the double bond that occurs in sp² hybridization and the triple bond in sp hybridization are formed by first establishing a single (sigma) bond, followed by the addition of pi bonds. Specifically, a double bond consists of one single bond plus one additional pi bond, while a triple bond consists of one single bond plus two additional pi bonds. The formation of these additional pi bonds is due to the interactions between the remaining p orbitals after hybridization. Thus, hybridization explains the formation of pi bonds.
While atoms involved in a single bond can rotate freely, as shown in the illustration, the presence of pi bonds makes rotation quite difficult. If one attempts to forcefully rotate the bonded atoms, the bond may break. Through hybridization, we gain a deeper understanding of how molecules bond with each other.
Comparison of sigma bonds and pi bonds
We have examined how the single (sigma) bond, a strong form of covalent bonding, and the pi bonds that form double and triple bonds are related to hybridization. Below is a simple comparison of the differences between sigma bonds and pi bonds:
And if we visualize the hybridization process along with a comparison of sigma (σ) bonds and pi (π) bonds by type of hybridization, it can be demonstrated as follows: