Hybrid Orbital Theory
Orbit vs. Orbital
The term "orbit" brings to mind the image of the solar system, where planets revolve around the sun in stable and defined paths. At one time, it was believed that electrons also revolve in fixed orbits around the nucleus. However, as research on electrons progressed, it became clear that due to their unique dual nature, electrons cannot be accurately described using the conventional concept of an orbit. With the advancement of quantum mechanics, the position of an electron is now represented by calculating the probability of finding it within a certain space, typically defined as the region where there is a 90% probability of its presence. This is known as the concept of an "orbital." The orbital represents a distribution where the electrons are expected to be found, resembling a cloud-like region. However, unfortunately, standard electron configurations have limitations when it comes to explaining the actual positions of electrons during the formation of bonds with other atoms. To address this limitation, a new theory was proposed: Hybrid Orbital Theory.
Electron shells and electron configuration of atoms
We have already examined in detail how electrons are arranged within atoms in other articles . To quickly recap, the nucleus is located at the center, surrounded by electron shells arranged according to specific energy levels (or principal quantum levels), designated as K, L, M, etc. Each of these shells is further divided into subshells, which include s, p, d, and f orbitals. These subshells, referred to as orbitals, represent the spaces where electrons are likely to be found, calculated as probability distributions. The first orbital within a shell, the s orbital, has a spherical probability distribution, while the next, the p orbital, is represented as having a dumbbell shape along three axes (x, y, z). This indicates that electrons are expected to be found within these dumbbell-shaped regions.
Each orbital can accommodate a maximum of two electrons, which occupy the same orbital with opposite spins (upspin and downspin). Electrons fill the shells in order, starting with the s orbitals before moving onto the p orbitals. Additionally, we discussed the octet rule, which states that an atom is most stable when it has eight electrons in its outermost shell. Atoms with fewer or more than eight valence electrons tend to interact with each other, either exchanging electrons or sharing them to achieve a stable octet. This is a very brief summary of electron configuration within atoms. For more detailed information, please refer to the previous articles.
2. Atoms: period(Electron shell and s,p,d,f orbitals)
3. Atoms: group(valence electrons and Ionic/Covalent/Metallic Bond)
The octet rule, which states that atoms tend to achieve a stable configuration by having eight electrons in their outermost shell, has limitations when explaining the bonding behavior of certain elements. Let's examine this using carbon's valence electron configuration as an example.
Carbon has an atomic number of 6, which means it has a total of 6 electrons. These are arranged as follows: 2 electrons in the 1s orbital of the K shell, 2 electrons in the 2s orbital of the L shell, and 1 electron each in the px and py orbitals of the p subshell. This configuration totals 6 electrons. While this ground state arrangement adequately describes the stable state of carbon, it poses challenges when explaining carbon's ability to form various bonds in organic compounds. Carbon is known to have four bonding "arms," meaning it typically forms four covalent bonds. However, based on its electron configuration, carbon appears to have only two unpaired electrons, which would suggest that it can only participate in two bonds. This limitation arises because the two unpaired electrons can only form two covalent bonds.
To overcome this limitation, a more creative approach is needed, leading to the development of hybridization theory. Hybridization allows for the mixing of atomic orbitals to create new hybrid orbitals that can accommodate more bonding possibilities, thereby explaining carbon's ability to form four covalent bonds with other atoms.
In chemistry, hybridization refers to the process of mixing two or more standard atomic orbitals to create entirely new types of orbitals. This new orbital is different from the original ones in terms of energy and shape. Both orbitals with a single electron and those fully filled with two electrons can participate in hybridization, as long as they have similar energy levels. When hybridization occurs, it results in the formation of new orbitals that have different energy levels and geometries. This concept is crucial for predicting the types of bonds, bond strengths, and molecular shapes, thereby aiding in the understanding of molecular geometry. Hybrid orbitals are classified based on the types and numbers of orbitals that are mixed. Common types include sp³, sp², sp, sp³d, sp³d², and sp³d³. In the cases of sp³d² and sp³d³, d orbitals are also involved in the hybridization process.
Why hybridization?
Since orbitals represent the spatial distribution of where electrons are likely to be found, based on wave functions, hybridized orbitals also represent the regions with the highest probability of finding electrons. However, the focus of hybridized orbitals is on how electrons are arranged to form bonds with other atoms. Through hybridization, the electron density can be concentrated in specific directions, enabling stronger and more stable bonds with other atoms. For example, in a standard s orbital, the electron distribution is spherical, meaning the electron density is uniformly spread in all directions without a specific orientation. In contrast, hybridized orbitals possess directionality and are concentrated, making them much more effective when overlapping to form bonds.
Additionally, hybridization allows for optimal geometric arrangements that minimize electron-electron repulsion, resulting in favorable bond angles. If one were to ask why hybridization is necessary, these advantages would provide the answer: it maximizes and optimizes the overlap of orbitals by concentrating electron density, thereby creating structures that allow for strong and stable bonding. Without these benefits, there would be little reason to complicate matters by mixing standard orbitals through hybridization. Therefore, the purpose of hybridization can be summarized as optimizing bonds and enhancing the stability and structural characteristics of molecules.
For reference, hybridization refers to the mixing of orbitals within the same atom. In contrast, when two atoms bond to form a molecule, the theory that probabilistically calculates and illustrates the distribution of electrons across the entire molecule is known as the Molecular Orbital (MO) theory. One similarity between MO theory and hybridization is that both involve the redistribution of electron density when two atoms bond. In MO theory, the overlapping atomic orbitals combine to form new molecular orbitals, where the electron density is spread over the entire molecule rather than being localized around individual atoms. This concept highlights how the behavior of electrons can change during the formation of molecular bonds, much like how hybridization modifies the arrangement of electrons within a single atom to facilitate stronger bonding. Overall, hybridization and Molecular Orbital theory explain the electron arrangements and distributions that occur at the atomic and molecular levels during bonding, making them complementary theories.
Determining the type of hybridization
Hybridization is closely related to bonding. Therefore, we will focus on understanding hybridization through carbon, a key element that forms organic compounds by bonding with various elements. Let’s explore the three most common types of hybridization for carbon: sp, sp², and sp³. Carbon, which forms the basic framework of organic compounds that are fundamental to life, can bond with hydrogen to create hydrocarbons or with atoms such as oxygen, nitrogen, sulfur, and halogens (F, Cl, Br, I) to form carbon compounds. Our bodies are composed of carbon compounds such as proteins, carbohydrates, and fats. Glucose, a vital energy source for life, is also a carbon compound. Furthermore, fossil fuels like coal and oil, as well as plastics, fibers, pharmaceuticals, and many other aspects of our world, are based on carbon.
One of the secrets to carbon's extensive versatility is hybridization. It is a key principle that enables the various bonding modes and structural diversity of carbon.
Hybridization of carbon
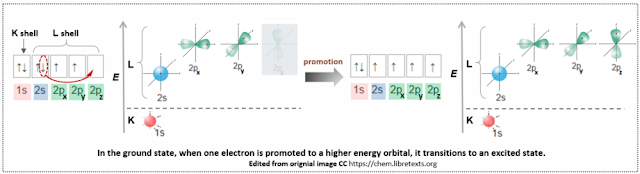
The ground state electron configuration of carbon is 1s2, 2s2,sp2. The 1s orbital in the first electron shell (K shell) is stable and does not participate in bonding, so only the 2s and 2p orbitals in the outermost shell (L shell) are involved. There are two electrons in the 2p orbitals, each occupying one of the two p orbitals (not paired), while one p orbital remains vacant. It's important to remember that s and p orbitals have different energy levels, and that the two unpaired p orbitals can participate in bonding.
Hybridization occurs when carbon seeks to bond with other atoms. Carbon can undergo various types of hybridization, and the specific type it adopts is determined by the number of partner atoms it wants to bond with and the type of bond being formed (single, double, or triple bond). For instance, if carbon forms four single bonds with four atoms, it requires four identical hybrid orbitals (sp³). When it forms a double bond with three atoms, three hybrid orbitals (sp²) are needed. Lastly, when carbon forms a triple bond with two atoms, it requires two hybrid orbitals (sp). The ability of carbon to tailor its orbitals according to the bonding requirements is truly remarkable, showcasing its creativity and flexibility in forming various structures.
Hybridization process of carbon
Hybridization is the process that resolves both the energy differences between orbitals and the issue of insufficient orbitals for bonding. First, to equalize the energy levels, one electron from the 2s orbital is promoted to an empty 2p orbital. This promotion is necessary because hybridization can only occur when the involved orbitals have the same energy level. As the electron is promoted, the energy levels of the orbitals become equal, with the new energy level being approximately somewhat between the original 2s and 2p orbitals. This process occurs across all types of hybridization: sp, sp², and sp³. At this point, the carbon atom is ready for hybridization.
Once the energy levels of the 2s and 2p orbitals are equalized in the excited state, carbon begins to undergo various types of hybridization depending on the situation. The three most representative types of hybridization for carbon are summarized as follows:
sp³ Hybridization
The type of hybridization varies depending on the number of atoms that are to be bonded. The most common and frequently seen type is sp³ hybridization, which forms only single bonds. The ratio of mixing between the s orbital and p orbitals differs for each type of hybridization; in the case of sp³ hybridization, the s orbital contributes 25%, while the p orbitals contribute 75%. This results in the formation of four identical hybrid orbitals after mixing, which is a straightforward process.
The newly created four hybrid orbitals then form four sigma bonds (σ bonds) with four surrounding atoms. It is very important to note that all these bonds are single bonds. Atoms that are connected by single (sigma) bonds can rotate freely, allowing for the formation of numerous stereoisomers that differ only in their three-dimensional arrangement. Stereoisomers will be discussed in a separate section.
sp² Hybridization
In sp² hybridization, three of the four orbitals with equal energy are mixed to create new hybrid orbitals. In terms of the contribution ratio, approximately 33% comes from the s orbital and 67% from the p orbitals. During this process, one p orbital remains unhybridized. This unhybridized p orbital contains one unpaired electron. When this unhybridized p orbital later forms a bond with another atom, it overlaps parallel to the p orbital of the other atom, resulting in the formation of a pi bond (π bond).
sp Hybridization
Finally, in sp hybridization, only two of the four orbitals are mixed to create two new hybrid orbitals. The mixing occurs with 50% contribution from the s orbital and 50% from the p orbital. The remaining two p orbitals each retain one unpaired electron and do not participate in the hybridization process. When these unhybridized p orbitals later form bonds with other atoms, they create two pi bonds (π bonds).
Now, let's examine how hybridized carbon forms sigma (σ) and pi (π) bonds, as well as the relationship between sigma bonds, pi bonds, and single, double, or triple bonds in detail in the next article.