2. Vertical columns: Group
We saw earlier that the horizontal period is classified according to the number of electron shells, and we looked very briefly at the order of filling the electron shells and orbitals with the number of electrons known from the atomic number. So what common characteristics do vertical columns have? The outermost electrons in the last shell, which directly participate in chemical reactions, are called valence electrons. That is, when electrons are filled one after another, the electrons in the last outermost shell which participate directly in chemical reactions are called valence electrons. For example, oxygen, with atomic number 8, fills 2 electrons in the first shell (K shell; 1s2) and 6 electrons in the second shell (L shell; 2s2, 2px1, 2py1 spz1). In this case, the outermost shell, the L-shell, contains a total of 6 electrons, so the number of valence electrons for oxygen is 6. In the periodic table, oxygen belongs to Group 16 and the number 6 in the one's place indicates the number of valence electrons. Elements in the same group share similar chemical properties, and this is largely due to the number of valence electrons they have.

Octet rule
Here we need to look at the Octet rule. If you remember the Latin word octo, which means 8, you can guess that it is related to 8. The Octet rule is a fundamental concept in chemistry, especially for atoms in the second period of the periodic table, and based on the observation that noble gases like Helium (He), neon (Ne), and argon (Ar) of group 18 are highly stable with full outer shells with 8 electrons and have very low reactivity with other surrounding atoms. This tendency for atoms to fill the outermost shell with 8 electrons to be in a stable state like an inert gas is called the octet rule. Helium has an atomic number of 2 and is in a stable state because its first shell is filled and full of 2 electrons.
Therefore, for oxygen, which belongs to Group 16, with 6 electrons in the outermost shell, it lacks 2 electrons to achieve the stable state of 8. It may seek to acquire 2 additional electrons from somewhere to achieve stability. Likewise, there may be atoms that are troubled by having excess electrons. For example, magnesium (Mg), with an atomic number of 12, belongs to Group 2. Being in Group 2 means that it has 2 valence electrons.
Then, in the case of group 16 oxygen, there are 6 valence electrons in the outermost shell, so it is 2 electrons short to achieve a stable state of 8. You will want to bring in two from somewhere and achieve stability. Likewise, there may be atoms that are troubled by having excess electrons. For example, magnesium (Mg), with an atomic number of 12, belongs to Group 2. Being in Group 2 means that it has 2 valence electrons. Looking at its electron configuration, the first(K) shell has 1s2, the second(L) has 2s2, 2px2, 2py2, 2pz2 and the third M shell has 3s2, totaling 12 electrons. If we visualize this, it would look like the diagram below. When there is a shortage of 6 electrons to reach 8, it might be better and easy to simply discard 2 electrons.
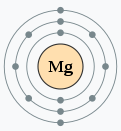
So how do we utilize the group from the table?
We now understand the concept that atoms may seek to acquire or discard electrons to achieve a more stable state based on the number of valence electrons. The group number indicates the number of valence electrons an element has and these numbers are crucial for predicting and understanding the chemical behavior of an element, such as predicting the types of chemical bonds that occur, including ionic bonds, covalent bonds, and metallic bonds.
1. Ionic bond
Ionic bonds primarily occur in groups 1 and 2, where elements tend to lose 1 or 2 electrons (alkali metals and alkaline earth metals), and in groups 16 and 17, where elements tend to gain 1 or 1 electrons (chalcogens and halogens). Since ionic bonds typically involve the transfer of electrons from metals(electron donors) to non-metals(electron acceptors), this bond is specifically called a bond between metal and non-metals. For example, sodium (Na), a metal in Group 1, can lose an electron to chlorine (Cl), a non-metal in Group 17 to form Sodium chloride (NaCl). When an element loses one outer electron, it becomes a positively charged cation, when gaining negatively charged anions. This attraction between the opposite charges is the force that holds ions together in an ionic bond.
2. Covalent bond
A covalent bond is a bond in which the two participating atoms share the electrons that they lack to achieve a more stable electron configuration. The sharing of electrons involves the overlapping of electron orbitals in the outermost shell of the participating atoms. The shared electrons are often represented as a bonding pair. In the example of a hydrogen molecule (H2), each hydrogen atom contributes one electron, and these electrons are shared between the two atoms. Both hydrogen atoms achieve a stable configuration with two electrons in their outermost shell. In another example of Hydrogen Sulfide(H2S), sulfur and hydrogen atoms share electrons to achieve stability.

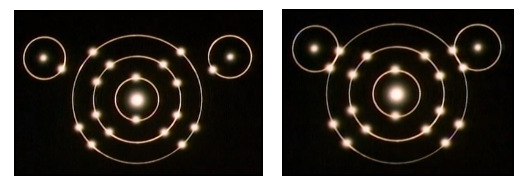
Unlike ionic bonds, covalent bonds do not involve the loss or gain of electrons. Covalent bonds are prevalent in molecules composed of non-metal elements.
Covalent bonds are illustrated by important elements such as carbon (Group 14), nitrogen (Group 15), and the chalcogens oxygen and sulfur (Group 16). Among these, carbon plays a fundamental role, particularly in the field of organic chemistry. Organic compounds, including essential molecules like hydrocarbons and biomolecules, heavily rely on carbon's ability to form multiple covalent bonds with other elements.
These elements, located in various groups of the periodic table, indeed play crucial roles in the formation of covalent compounds. The ability to share electrons among atoms enables the formation of a wide range of complex molecular structures. Covalent bonds, particularly prevalent in organic compounds, highlight the fundamental significance of carbon, nitrogen, oxygen, and sulfur in the intricate chemistry that underlies the phenomena of life.
3. Metallic bond
In metallic bonding, the electrons from the outermost shell of a metal are liberated from individual atoms, enabling them to freely move around the atoms without being restricted by the atomic nucleus. Let's take zinc as an example: in its metallic state, zinc exists as divalent cations, having lost two electrons. When a significant number of these zinc atoms come together, a multitude of electrons becomes detached, forming a dynamic sea or cloud of electrons, as depicted below.
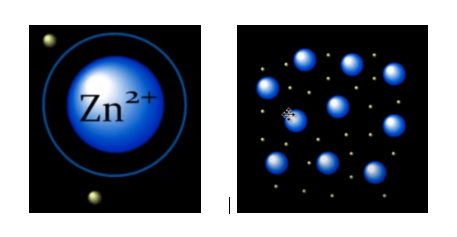
In this collective arrangement, all zinc cations actively participate in electron sharing, and it is important to note that these electrons are not specifically bound to any individual cation. In this state of unrestricted movement, the electrostatic attraction between the positively charged zinc cations and the surrounding cloud of negatively charged electrons binds the zinc cations together, forming a cohesive, three-dimensional structure that gives metals their characteristic solid form.
A solid form of metal
Free electrons and characteristics of metals
This bonding characteristic that is not fixed but moves fluidly explains various properties of metals. Because electrons move freely, they can be a good conductor of electricity. In addition, since the particles can all move freely, if pressure is applied to one side, all the particles move in that direction and become bent(malleability). Because the metal cation layers can slide over each other without breaking the metal bonds, they can be made thin by hitting them with a hammer like in a blacksmith shop. The presence of free electrons also contributes to the reflective nature and luster of metals. Metallic bonding is a strong type of bonding, requiring significant energy to break, which is why metals generally have high boiling and melting points.
The Beauty of Metallic Bonding: Alloys
The creation of alloys is the profound contribution of metallic bonding. The exceptional mobility of metal particles allows for a seamless integration with other metals and materials. Throughout history, we have continuously enhanced the hardness, durability, and practicality of metals through the process of alloying. It has become an essential aspect of our daily lives, with countless examples. From the moment humans first crafted bronze by combining copper and tin, the journey of upgrading our lives has never ceased. We have fortified iron into steel through the union with carbon I can't even imagine my kitchen without these corrosion resistant alloys. I would like to give a special applause to Chromium. I would like to point out again that all of this would not have been possible without the characteristics of metallic bonding.
Until now, I've delved into the world of atoms, the most basic unit of matter. The purpose of exploring the concept of the electron shell and the count of valence electrons, expressed in the periodic table, is clear. It was a preparatory warming-up exercise before the attempt to comprehend and appreciate the various phenomena that occur to us. From the numerous organic substances surrounding us to the intricate processes of life such as breathing, walking, digesting, and creating energy, we cannot understand the causes and effects of reality without understanding the principles of atoms since they are the building blocks of matter, and their behavior at the atomic and subatomic levels influences the macroscopic world we observe. Of course, if we explore deeper and broaden our approach, it will become much more complex with numerous exceptions, but I think I have sufficiently grasped the most fundamental framework.
Starting with this understanding as a first step, I plan to take the next step and explore further. I will continue to study how electrons actively participate in our lives, particularly within the context of our human bodies.





