I'm going back to the beginning and study atoms again.
When reading papers and articles related to the human body or its functions, I frequently encounter challenges, particularly in the area of chemistry, especially basic chemistry. I admit that I neglected and didn't pay sufficient attention to it, trying to get away from it as much as I could without a deeper understanding. Now, I am paying the prices. Is this a form of karmic consequence?
Take "oxidation" as an example, which I hear almost as often as my pet's name whenever I read health-related articles. "Oxidation is harmful. Oxidative stress is the root cause of all diseases.. etc." It feels like a repetitive chorus of a famous song, and I find myself wanting to understand the mechanisms behind oxidation and oxidative stress in detail. Not just from an academic perspective, but for practical understanding as well. Understanding the process of oxidation and its potential consequences can only help us to get insight into antioxidants, which are a defense mechanism against their harmful effects. Various chemical reactions, chemical formulas, and especially the various functional groups related to metabolism that we see every time we read numerous research papers are difficult to understand and give me a headache. I really need to understand and more importantly, I really want to comprehend. So, I plan to study the basics and write down what I learned whenever I have time. The fact that I can write it down proves that I understand completely. There is no way to write about something without truly understanding it. Once again, I find myself walking down the rabbit hole of Wonderland. Excited, anxious, but hopeful.
Understanding oxidation is a great help in understanding the cellular respiration process. This respiration process, which creates the energy we need, involves coenzymes and chemical bonds. But the most important thing is the movement of electrons. It is through this process of losing and receiving electrons that we maintain life. In the early days, oxidation was defined as the process of obtaining oxygen, but it was later expanded to include the loss of electrons to explain oxidation reactions that occur even without the involvement of oxygen. If our bodies function through the gaining and losing of electrons, in other words, oxidation and reduction reactions, then it seems that we would eventually have to go back to atoms from the beginning with a humble attitude. Fascinated by the mysteries of the human body's operations, I made up my mind to return to the basics and start from scratch, here I mean "atom".
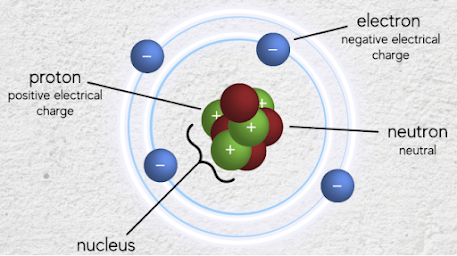
The members of an atom
An atom is widely known as the fundamental particle of a pure substance that cannot be further divided. To put it simply, an atom has a nucleus in the center and electrons revolve around it. According to what is known and revealed to date, the atomic nucleus is made up of protons and neutrons, each made up of three quarks. An atom is electrically neutral only when the number of protons with a positive (+) charge and the number of electrons with a negative (-) charge are equal, so the number of protons and electrons in each atom is the same. And this number becomes the unique atomic number of that atom.
Beryllium atom, atomic number 4, with 4 protons and neutrons in the nucleus and 4 electrons.
To overcome the strong repulsion between positively charged protons within the atomic nucleus, neutrons coexist with protons within the nucleus to help bind and keep them together. This may be the reason why hydrogen, which has only one proton, does not have any neutrons. Hydrogen, the first element formed along with the Big Bang, still accounts for 90% of the universe. Hydrogen has an atomic number of 1, with only one proton and one electron, making it the simplest atom. Since it easily loses its electron, it is also called a hydrogen proton. However, from oxidation to cellular respiration, hydrogen's role is truly remarkable. The more I study, the more I realize that it is a truly pivotal atom.
Periodic table and me
I remember my chemistry teacher advising us to memorize the periodic table during my high school days. We were already memorizing lengthy whole classic poems anyway but that idea came as shocking and even daunting. It's a shame that I didn't deeply appreciate the profound principles and secrets contained within the periodic table at that time. If I had understood the meaning conveyed by the rows and columns of the periodic table, it would have been easier for me to comprehend the big picture of the substances that make up our world. The periodic table also serves as a key to unlocking the mysteries of chemical bonds. Metallic, ionic, and covalent bonds, the trio responsible for creating a multitude of substances, find their explanations within the structured layout of the periodic table. With this understanding, the reasons behind elements' tendencies to combine and the mechanisms of their combinations become clearer. Fortunately, common sense exists in science as well.
Periods and Groups
The periodic table is arranged based on the concept of periods and groups. As the atomic number increases, elements with similar properties appear periodically. The vertical columns (groups) classify elements based on the number of electrons in the outermost electron shell, while the horizontal rows (periods) classify elements based on the number of electron shells they possess. (Well, some people have worked so hard to articulate this rule, then I should at least be able to understand it. 😅) From my limited knowledge of chemistry, electrons seem to play a central role in chemistry, shaping everything in the world of chemistry. They determine the nature of an atom, participate in the creation of new compounds through the exchange of electrons, and facilitate electron transfer and movement. Therefore, it seems that everything is related to electrons.
In the following article, I will look at the period and the groups in more detail one at a time.