Three major sites where ROS is produced in the human body are mitochondria, white blood cells, and uric acid excretion.
Cellular respiration: Mitochondria, the scene of a delivery accident
Through a seemingly trivial(?) process of losing electrons, we create the energy necessary to sustain life. This remarkable process primarily occurs within the mitochondria. Let’s take a very brief look at the process the human body goes through to produce energy called ATP(Adenosine triphosphate). After nutrients are indigested through food, they go through several processes until they are converted into pyruvate. This pyruvate then enters the mitochondrial matrix, triggering a cycle called the TCA cycle(Tricarboxylic acid cycle). This is the process of converting the energy stored in molecules like NADH and FADH2 into ATP so that the human body can utilize it. This process of conversion occurs in the electron transport chain(ETC) in the inner membrane of the mitochondrial matrix within cells. This electron delivery system consists of four protein complexes and two electron transport carriers. Here, NADH and FADH2 release their electrons, which are then transferred through numerous redox centers within the complexes. These electrons are finally delivered to the oxygen molecule, which combines with the surrounding hydrogen to create water. You might wonder If you were going to deliver it to oxygen anyway, why not just give it to them directly? Why do we need to take such a complicated and long-winded step to transfer electrons? There is a reason. Each time electrons are transferred, that is-, energy is released. A significant amount is released. If the amount were too large for our bodies to handle, it would be overwhelming. Therefore, to gradually release the energy in manageable amounts, it is transferred in a circuitous route, taking detours instead of going directly.
So, is the energy generated by transferring electrons the same energy that our human body uses for life activities? Not like that. The energy generated by transferring electrons is actually used to push hydrogen out of the inner membrane of the mitochondria. Mitochondria is surrounded by two membranes, inner and outer membranes. And intermembrane space between these two can be thought of as a moat that surrounded a castle in the Middle Ages. ETC exists in the inner membrane attached to the substrate. When NADH and FADH2 release electrons, they also release hydrogen ions, and that energy generated when giving up electrons is used to pump and expel these hydrogens into the intermembrane space(moat). As hydrogen ions accumulate in this intermembrane space, creating a significantly higher concentration than within the mitochondrial matrix, a chemical gradient is formed. This gradient drives the hydrogen ions back into the mitochondria. This driving force is what produces ATP, the energy currency used by the human body. This movement of hydrogen ions is similar to water flowing down a water wheel or water falling from a high place. ATP is generated in a similar way by the force that rotates a water wheel as water pours down, or the potential energy of water falling from a high place in a dam to turn a turbine. For ADP(Di = 2), which has two phosphate groups, to become ATP (Tri = 3), one more inorganic phosphate (P) must be combined, and the energy required to form this combination is obtained using the concentration difference of hydrogen protons. I plan to post in detail the process of energy generation for human use, known as cellular respiration. The reason these processes are discussed here at length is because they result in the production of reactive oxygen species (ROS) in this vital phenomenon of daily life. It means that it is unavoidable and inevitable for some electrons to get lost, misplaced, or leaked elsewhere, despite efforts to meticulously transfer electrons to oxygen. Such "delivery accidents" frequently occur, where electrons are not delivered to oxygen, the intended destination, and is instead leaked to the wrong place. The electrons discharged in this way become the main characters that cause oxidation.
Original image: https://microbenotes.com/electron-transport-chain/
We will learn more about radicals in the next article. For now, let us mention that mitochondria are an environment in which radicals from superoxide radicals to hydrogen peroxide can be easily generated due to the abundant presence of the ingredient such as leaked electrons from ETC, a large number of hydrogen protons, and oxygen molecules. It is estimated that our human body has approximately an average of 30 trillion cells [1]. And within one cell, there are approximately 1,000 to 2,500 mitochondria, although there may be some differences depending on the body part [2]. ATP production through cellular respiration accounts for 90% of total ATP, and ATP must be constantly produced since once ATP is produced, there is no way to store it. So, how much ATP is produced inside mitochondria per day? Considering that one cell utilizes around 10 billion ATP per day, an adult requires approximately 3.0 × 10^25 ATP daily. The ATP energy produced by a healthy adult per day is converted to 1,200 watts. It is a truly remarkable activity that cannot be underestimated. These figures highlight the scale of electron leakages and loss that inevitably occurs in this vast and enormous process of producing ATP. It indicates that the generation of reactive oxygen species can occur on a larger and more frequent scale than we think.
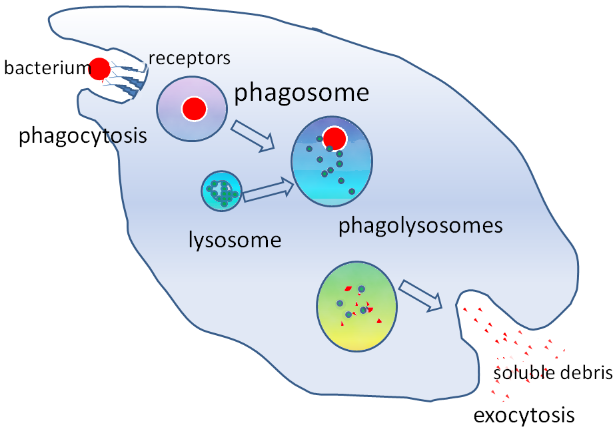
Respiratory burst: phagocytes, bullets of white blood cells fighting invaders
When microorganisms, including bacteria, fungi, and viruses, invade from external sources, phagocytes and neutrophils within white blood cells are the first to respond and arrive at the scene as the primary defense line of the immune system. Upon engulfing the pathogens, they generate and utilize reactive oxygen species as weapons to effectively and efficiently destroy and eliminate the pathogens. Phagocytosis is initiated by the activation of an enzyme called NADPH oxidase, which plays a pivotal role in the immune response against pathogens. This process, often referred to as a "respiratory burst", is a crucial mechanism of the innate immune system, vital for host defense and immune regulation. Further details on this respiratory burst phenomenon will be elaborated in a separate article.
Uric acid excretion from the body: the final step in purine metabolism, a component of nucleic acid
DNA, a polymer complex compound that contains the genetic information of living organisms, is composed of purine bases and pyrimidine bases.
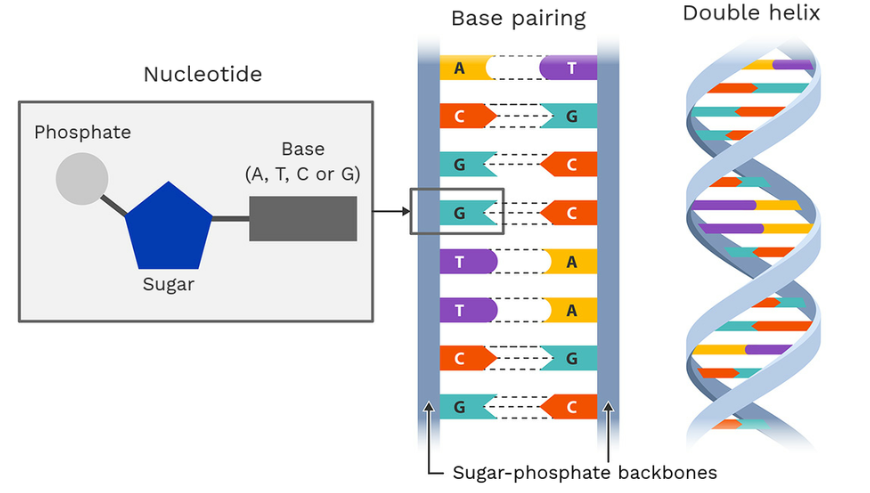
The basic unit of DNA and RNA is nucleotides, which are organic molecules composed of a nitrogenous base, a pentose sugar, and a phosphate. In the case of DNA, pentose sugars and phosphates are alternately connected to form a backbone on the outside on both sides, and bases are connected to each other on the inside. When connected according to the carbon positions in the phosphate, it takes on a twisted helix shape. While DNA has two strands, RNA is a single strand. There are two types of nitrogen bases in nucleotides: Purine and Pyrimidine. Purine bases include adenine and guanine, and pyrimidine bases include cytosine, thymine (for DNA), and uracil (for RNA).
Uric acid is the final metabolic product of purine. Since the human body cannot further break down uric acid, it must be excreted in the form of urine. When cells die or are damaged, their DNA is released into the blood and degraded. During this process, purine is converted to Hypoxanthine, which is then converted to Xanthine by Xanthine Oxidoreductase (XOR). Finally, Xanthine is converted to uric acid by this same enzyme. This uric acid is then transported to the kidneys and expelled from the body through urine. This is the mechanism by which uric acid is eliminated from the body
In all organisms, Xanthine Oxidoreductase (XOR) typically exists in the form of a Dehydrogenase known as Xanthine Dehydrogenase (XDH). However, only in mammals, it can be converted to its oxidized form, Xanthine Oxidase(XO). XDH utilizes NAD+ as a cofactor to accept electrons and is reduced to NADH. During this process, the generation of reactive oxygen species (ROS) is minimal. On the other hand, XO directly transfers electrons to oxygen molecules, resulting in the production of superoxide anion radicals (O2•-) through single-electron transfer and hydrogen peroxide (H2O2) through two-electron transfer. Moreover, when these reactive oxygen species (ROS) interact with surrounding iron or other transition metals, they can even generate hydroxyl radicals via Haber-Weiss and Fenton reactions. XDH conversion to XO is observed in various pathological conditions, particularly in hypoxic and ischemic conditions such as organ transplantation.
When uric acid accumulates in the body beyond an appropriate amount, the poorly soluble molecule forms needle-shaped crystals. These accumulated crystals can form in the kidney tubules, leading to the formation of kidney stones and additionally, in the joints, resulting in a condition known as gout.
We have examined the three most representative mechanisms by which reactive oxygen species are generated. Regardless of whether they have positive or negative effects, it is very important to understand that their generation is a very ordinary part of life. In other words, it is essential to recognize that reactive oxygen species are not produced through any extraordinary mechanism but rather occur naturally in our everyday lives.
[Reference materials]
[1] Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4991899/
[2] Pizzorno J. Mitochondria-Fundamental to Life and Health.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684129/
[3] Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects