In the previous article, we looked at the most representative mechanisms by which active oxygen is created in the human body, including NADPH oxidase catalytic activity for immune reactions, intracellular oxidative phosphorylation for ATP production, and the uric acid excretion pathway. All of these are necessary responses to preserve our healthy lives. Our human body, which needs powerful lethal weapons to kill germs, including bacteria invading from the outside, utilizes the NADPH oxidase complex to produce various reactive oxygen species. This is called a respiratory burst or oxidative burst. Let’s take a closer look at the radical production process to dissolve bacteria engulfed by phagocytes .
Phagocytosis of white blood cells, the first line of defense.
First, let's briefly explore the process of phagocytosis carried out by Macrophages and Neutrophils, which are white blood cells involved in the respiratory burst. Neutrophils and Macrophages, constituting up to 70% of white blood cells, serve as the front line of defense in innate immunity, swiftly responding to external invaders such as bacteria or viruses. Upon encountering a pathogen, they move towards it without delay and engulf it entirely, and form a phagocytic sac by surrounding it with a Phagosome, a round vesicle filled with fluid. Subsequently, phagosomes fuse with Lysosomes, which contain enzymes capable of hydrolyzing and digesting pathogens, forming Phagolysosomes that produce reactive oxygen species(ROS), which can dissolve and kill pathogens. Within the Phagolysosome, the pathogens are broken down and processed, with useful molecules such as amino acids sent to the Cytoplasm for recycling, while water-soluble waste is expelled from the cell. Once their task is complete, the Phagolysosome is dismantled.
NADPH oxidase
During this process, NADPH oxidase within the phagolysosome produces ROS and performs destructive antibacterial activity that kills pathogens and viruses.
NADPH and NADPH oxidase
NADPH(Nicotinamide Adenine Dinucleotide Phosphate) is a coenzyme involved in intracellular redox reactions, serving as an electron transfer medium that carries and transfers high-energy electrons. It acts as an important cofactor in numerous biochemical reactions, primarily functioning as a reducing agent by donating electrons to other molecules. Similar to the NADH(Nicotinamide Adenine Dinucleotide) coenzyme, which participates in catabolic processes generating energy such as glycolysis, the TCA cycle, and oxidative phosphorylation(ETC), NADPH is derived from vitamin B3 niacin. Both NADPH and NADH play essential roles in cellular metabolism and redox balance
The NADPH oxidase enzyme is an enzyme complex that utilizes NADPH as an electron donor to transfer electrons to oxygen, thereby reducing it and catalyzing the generation of reactive oxygen species (ROS) such as superoxide radicals and hydrogen peroxide.
NOX Family
The NADPH oxidase(NOX, Nox) family consists of seven members, including Nox1 to Nox5, as well as dual oxidase enzymes Duox1 and Duox2(also known as Nox6 and Nox7, respectively). Each member of this family exhibits distinct functions and activation properties and is widely distributed across various cells and tissues. Examples of their roles include host defense(Nox2), otolith formation in the ear(Nox3), iodination of thyroid hormones(Duox2), and regulation of vascular tone (Nox2) [2]. NOX proteins have also been shown to be involved in the regulation of many basic physiological processes, including cell growth, differentiation, apoptosis, and cytoskeletal remodeling.
Then, what is the role of ROS generated by NOX? It can be summarized into two main roles. Firstly, they serve as traditional phagocyte weapons for host defense, a function that has been well-established for a long time. Secondly, they are involved in signal transduction, a role that has gained attention more recently. ROS produced through NOX can interact specifically and reversibly with proteins, modifying their activity, localization, and stability. In non-phagocytic cells, ROS often regulate signal transduction by modulating the activities of kinases and phosphatases, as well as gene transcription. Unlike superoxide radicals, which have a negative charge and face difficulty in crossing biological membranes, hydrogen peroxide (H2O2) can permeate membranes and is relatively stable. Thiol oxidation by H2O2 has been implicated in various signaling pathways, leading to diverse physiological outcomes. The role of H2O2 as a signaling molecule is discussed in more detail in another article. However, the primary function of NOX is the generation of reactive oxygen species.
Mechanism for producing reactive oxygen.
Flavocytochrome b558 complex and four subunits
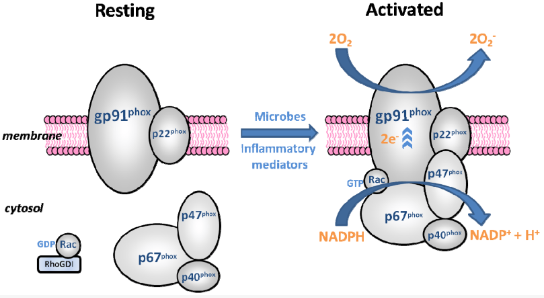
Let's look at the components of the phagocytic NADPH oxidase complex. At the center of this catalytic complex is the glycoprotein gp91phox. Phox is an abbreviation for phagocytic oxidase and refers to phagocyte catalytic enzyme. gp91phox is anchored to the cell membrane and is better known by its nickname NOX2. It combines with the smaller p22 phox protein and forms the flavocytochrome b558 complex(i.e., gp91phox + p22phox), which can be considered the key component and central element of this catalytic activity.
In addition to the flavocytochrome b558 complex, another group of subunits is separated from this complex and exists in the cytoplasm rather than in the membrane. There are four subunits: p47phox, p67phox, p40phox, and the cell signaling G-protein Rac and Rap1A GTPases. They exist in a stable state in the cytoplasm during the resting phase, and only when the catalyst is activated in response to stimuli such as bacterial or fungal infection, inflammatory cytokines, and other signaling molecules do they move directly to the membrane and dock with flavocytochrome b558. The physical and spatial separation between these two groups can be said to be our human body's meticulous efforts to prevent excessive and uncontrolled production of ROS. In fact, p40phox and p47phox are originally in a self-inhibited state, so they must go through a phosphorylation process to become activated. This complex system that ensures the assembly of oxidase subunits and the formation of an active enzyme complex only when appropriate signals are received appears to have evolved over a long period of time.
Generation of superoxide radicals
Once all six units are combined and assembled around the flavocytochrome b558 complex in the Phagolysosomal membrane, preparations are complete to begin producing active oxygen. At this stage, the complex receives electrons from NADPH in the Cytoplasm and transports them within flavocytochrome b558 through six helical structures that alternately traverse the inside and outside of the cell membrane. NADPH, which catalyzes this reaction, releases two electrons and one hydrogen proton and is oxidized to become NADP+. While the proton remains in the Cytoplasm, the two electrons are transported through the phagocyte membrane, ultimately binding to two oxygen molecules and producing two superoxide radicals in the extracellular or intracellular phagocyte space. These radicals serve as a powerful antibacterial agent used to destroy pathogens captured by phagocytes.
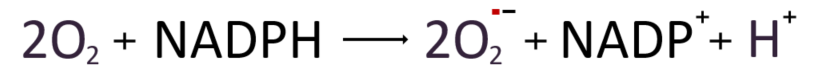
Production of hydrogen peroxide (H2O2)
The generated superoxide radical then can be converted into hydrogen peroxide and water either by naturally acquiring electrons from the surrounding environment, or through the intervention of superoxide dismutase (SOD).
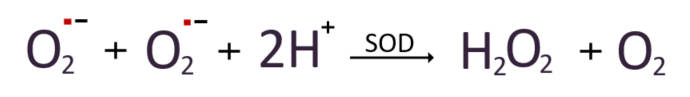
Various antioxidant enzymes convert hydrogen peroxide into safe water. The two most well-known examples include Glutathione Peroxidase(GPx) and Catalase.

More powerful ROS: hypochlorous acid and hydroxyl radical
Hydrogen peroxide can indeed be converted into safe water when it encounters antioxidant enzymes. However, if it combines with chloride ions (Cl-), it generates hypochlorous acid, which has a strong insecticidal effect. This process is catalyzed by an enzyme called Myeloperoxidase (MPO). Hypochlorous acid is a potent pesticide and is also an ingredient used in household laundry bleach. While hypochlorous acid can be a reliable ally in eradicating invading pathogens, MPO leaked out of cells can often produce hypochlorous acid inappropriately. This can lead to damage to host tissues and promote disease development, especially in chronic inflammatory reactions. Thus, MPO-mediated generation of hypochlorous acid can have negative effects and cause problems.
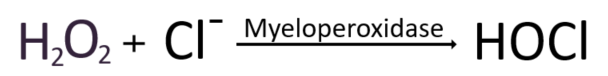
A variety of reactive oxygen species (ROS), including superoxide radicals, hydrogen peroxide, and hypochlorous acid, are generated to facilitate the removal of bacteria by phagocytes. However, the process doesn't end there, as even more potent and lethal ROS, such as hydroxyl radicals, can be produced. When iron or copper cations are present nearby, hydrogen peroxide reacts with them to form hydroxyl radicals. This reaction is known as the Fenton reaction and the Haber-Weiss reaction.
The production of reactive oxygen species (ROS) is a mechanism in our body's immune response to defend against harmful pathogens. However, if various types of ROS are excessively produced beyond what is necessary, the balance between reactive oxygen species and antioxidant enzymes can be disrupted, leading to oxidative stress. Therefore, even though intentional ROS production serves a beneficial purpose, the role of antioxidant enzymes in controlling and regulating them is crucial.
NOX Gene Mutation Chronic Granulomatous Disease (CGD)
We have examined the antibacterial immune response mediated by ROS, with NADPH oxidase as the starting point of this reaction. The significance of normal expression of this enzyme for immunity can be understood from the severe genetic disorder known as Chronic Granulomatous Disease (CGD).CGD is a genetic immune system disorder where NOX fails to produce ROS normally due to genetic mutations in proteins like gp91phox, which are components of NOX. Consequently, the immune system is unable to effectively kill pathogen and instead tries to surround them with multiple layers, leading to the formation of blood cell clumps and granulomas. However, this attempt is not as effective as ROS and life-threatening infections constantly recur, and can eventually lead to fatal outcomes. This is an example showing that ROS plays an indispensable central role in the immune defense system.
[Reference materials]
[1] NADPH oxidases: an overview from structure to innate immunity-associated pathologies
https://www.nature.com/articles/cmi201489
[2] Nox proteins in signal transduction
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2763943/
Defining the molecular role of gp91phox in the immune manifestation of acute allergic asthma using a preclinical murine model.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3266200/
Activation and assembly of the NADPH oxidase: a structural perspective
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1134858/
NADPH Oxidase as a Therapeutic Target for Neuroprotection against Ischaemic Stroke: Future Perspectives
https://www.mdpi.com/2076-3425/3/2/561
NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology
https://www.mdpi.com/2076-3921/10/6/890
Chronic Granulomatous Disease
https://emedicine.medscape.com/article/1116022-overview?form=fpf