The root of all diseases?
When I read papers or research reports related to diseases, I frequently encounter the term "free radicals." Free radicals are even implicated in speeding up aging by damaging telomeres. What are they? I'm curious. What exactly are free radicals? I know they're often portrayed as villains, but I want to understand why they're considered harmful in more detail. Reactive oxygen. Let's begin by understanding what "reactive" means.
What is "reactive"?
The state in which a substance is not stable and can easily react with other substances is called a reactive state or radical. Atoms in a stable state have a low energy level and are less active. Then why did it become active? In the previous articles, we looked at the rules for arranging the electrons of atoms into orbitals. We also saw that each orbital can have up to two electrons. Electrons like to move in pairs. However, if one electron is lost or taken away for some reason, it loses its pair and becomes very unstable. It then seeks to replenish the lost electron, disrupting stability and tranquility. The term "radical" often brings to mind the image of something aggressive and uncontrolled. Reactive oxygen is associated with the loss of electrons.
Definition of radical
Molecules that contain one or more unpaired electrons are referred to as radicals. [1] Normally, electrons exist in pairs. When elements combine to form substances, they do so through various chemical bonding methods. In the case of covalent bonding, for example, elements share electrons to form pairs. This explains why elements with even atomic numbers do not become radicals—they can easily form stable pairs. Even when a molecule is split apart, the electrons remain paired and do not disintegrate. However, when a molecule is split abnormally, the stable covalent bond is broken and divided into two halves. This results in two unpaired electrons that cannot form a pair. This is a radical.
The terrifying characteristic of radicals: seriality
To restore stability by finding a new partner, a radical seeks out and attacks other molecules in its neighborhood, stealing electrons from them. In this way, it satisfies its own need for stability. However, this creates a new problem: the molecule that just lost its partner suddenly becomes a radical itself. Thus, a radical gives birth to another radical. This newly formed radical will immediately attack another nearby molecule, stealing its electrons to fill the void left by its lost partner. This chain reaction happens rapidly and within a short period.
When the initial radical is formed, it is called the "initiation phase." The continuous occurrence of radical reactions, where electrons are stolen successively, is known as the "propagation phase." This repetitive attack of radicals on non-radicals happens in a chain-like fashion, repeating countless times. Eventually, when the concentration of radicals is high enough, the radicals will react with each other and form stable non-radical compounds. This marks the end of the chain reaction, known as the "termination" phase. The chain nature of radical reactions is perhaps the most alarming characteristic of radicals. In reality, these destructive chain reactions can occur within our bodies. One example is the lipids oxidation that takes place in cell membranes, as mentioned in the previous article about AGEs. If a continuous chain reaction of radicals persists in a particular location for an extended period of time, it can lead to severe consequences, possibly resulting in fatal outcomes.
Oxidation and reduction (Redox)
In chemical reactions, when an atom or molecule loses electrons, combines with oxygen, or loses hydrogen, it is referred to as oxidation. Originally, oxidation referred to the process of combining with oxygen, as implied by its name, but it has been discovered that oxidation can occur even without the presence of oxygen. It can be said that "losing electrons" is a more general definition. On the other hand, reduction is the opposite process of oxidation. It involves gaining electrons, separating from oxygen, or combining with hydrogen. Oxidation and reduction often occur together as a pair. If there is someone losing electrons, there must be someone gaining them, as it is a natural balance. Therefore, it can be understood that reactive oxygen species are generated in the process of redox reactions (reduction and oxidation) where electrons are lost and gained.
“Losing electrons” – a prerequisite for energy production
"It is truly remarkable to see how important this seemingly simple event is. Life is sustained through this trivial process of electron transfer. Let's briefly take a look at a typical example: After nutrients from food are ingested, they undergo several processes until they are converted into pyruvic acid, which then enters the mitochondria triggering the repetitive cycle known as the TCA(tricarboxylic acid) cycle, also known as the Krebs cycle. The process of converting the energy stored in the carrier, NADH and FADH2 into ATP, the primary energy currency of the cell occurs in the electron transport system(ETS) located in the inner membrane of the mitochondrial matrix within cells.
In this process, electrons from NADH and FADH2 are continuously transferred through numerous subunit proteins and protein complexes, following intricate pathways. Finally, when they reach the oxygen molecule, oxygen accepts the electrons and combines with surrounding hydrogen to form water. One might wonder why electrons aren't transferred directly to oxygen if that's where they're headed. There is a reason for this. When electrons are lost, energy is released — a significant amount of energy. What if this energy were released all at once, beyond what our bodies could handle? It would be overwhelming. Therefore, to release the energy gradually, in manageable increments, it is delivered in a circuitous route rather than directly.
In human biology, the energy generated by transferring electrons is not directly used by the body. Instead, it is utilized to pump hydrogen ions from the inner membrane of the mitochondria to the outer membrane. The accumulated hydrogen in the intermembrane space, which is much more concentrated than the mitochondrial matrix, creates a significant concentration difference between these two areas. This difference in concentration namely concentration gradient allows the hydrogen ions in the inner membrane to be pushed back into the mitochondria through a special enzyme complex, called ATP synthase, producing ATP, which is the energy used by the human body. This process is similar to that of other energy-generating techniques such as a water wheel turned by flowing water or the principle of using the potential energy of water falling from a high place in a dam to rotate a turbine. To convert ADP, with two phosphate groups, into ATP, with two phosphate groups, an additional inorganic phosphate must be attached, and the energy required for this bond formation is derived from the concentration difference of hydrogen ions. I hope to post more detailed information about this ATP energy production process of cellular respiration at another time. The reason for mentioning these processes here is that a large amount of reactive oxygen species is produced in the middle of these biological events. This means that it is an inevitably occurring process. Despite efforts to efficiently transfer electrons to oxygen, some electrons inevitably stray or leak, resulting in oxidative stress. There are frequent incidents where electrons leaks to unintended locations, instead of being delivered to the waiting oxygen at the destination. This misdelivery itself is a natural part of biological processes and these stray electrons become the main culprits of oxidation.
Transformation of oxygen into reactive oxygen species
Now that we have examined what radicals are and what makes them reactive, let's take a look at oxygen. First, let's explore the processes through which stable oxygen transforms into reactive oxygen species. Reactive oxygen species come in various forms, and they are collectively referred to as Reactive Oxygen Species (ROS). We will discover that there are correlations among these species.
An oxygen atom
An oxygen atom, with an atomic number of 8, has 8 electrons. Applying the electron arrangement rules based on the shell (principal quantum number) and orbitals discussed in the previous postings, oxygen shows the following structure. In the first shell (K shell), which is closest to the atomic nucleus, there are 2 electrons in the s orbital. In the second shell (L shell), there are again 2 electrons in the s orbital, and in the p orbital, there are 3 orbitals in three different directions: px with 2 electrons, py with 1 electron, and pz with 1 electron, making a total of 8 electrons. The two orbitals have unpaired electrons and exist alone.
Electrons are considered to be in a stable state when they exist in pairs. However, oxygen has one unpaired electron in each of its two outermost orbitals, py and pz. Here, if we briefly recall the octet rule, which states that atoms tend to achieve stability by having 8 electrons in their outermost shell, oxygen requires 2 more electrons to satisfy the octet rule.
Triplet oxygen
Two oxygen atoms, each with the electron configuration described above, form a covalent bond to create O2, the triplet oxygen we breathe.
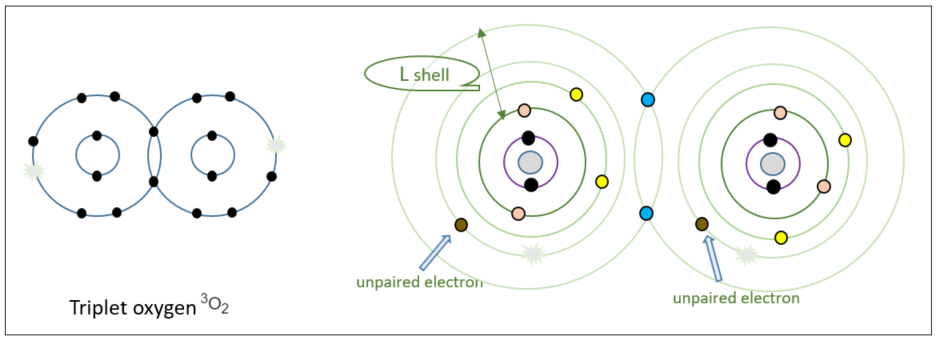
By sharing the electrons in the outermost orbit with each other, that orbit is filled with electron pairs. However, there is still an orbit containing unpaired electrons, not satisfying the octet rule. Normally, this configuration would be considered unstable due to the lone, unpaired electrons. However, oxygen is an exception to this rule and remains relatively stable in this state, despite not all electrons being paired. This stability allows us to breathe and sustain life. But what happens when this stable triplet oxygen acquires (or reduces) an electron from its surroundings?
Superoxide anion radical
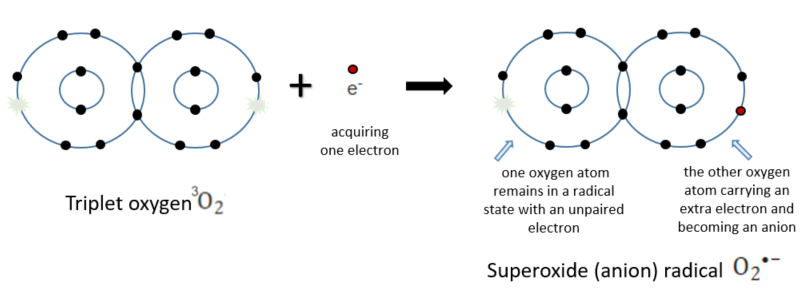
When we simplify the electron configuration of the L shell and express it visually, it appears as shown above. While one atom gained an electron (was reduced) to achieve eight electrons, satisfying the octet rule, the other atom still has one unpaired orbital remaining. Now, the question arises: Why not acquire two electrons at once? This is because oxygen molecules are triplet species capable of acquiring only one electron at a time. This results in the formation of a molecule with unpaired electrons, known as a radical. The remaining unpaired electron will make every effort to find a partner and form a pair, thus exhibiting high reactivity. The term "anion" indicates an overall charge of (-) because it has gained an electron with a charge of (-). In the study of chemistry, complex and lengthy chemical names may initially appear daunting. However, once you understand the naming conventions, it becomes easier to comprehend their properties. One more subject to study... By the way, radical molecules are indicated by placing a dot in their chemical formula
Starting from an oxygen atom, we encounter the first radical, known as the superoxide radical or superoxide anion in Korean. Superoxide radicals can react with various compounds to produce diverse compounds, as is typical of radicals. However, the main issue is their role in generating the well-known reactive oxygen species (ROS). ROS are highly reactive oxygen-containing compounds. It's important to note that while superoxide itself is a radical, it serves as the precursor for generating hydrogen peroxide by acquiring one more electron and combining with a hydrogen proton. Additionally, it is even more important to note that it also serves as a precursor to the generation of the most potent and harmful hydroxyl radical.
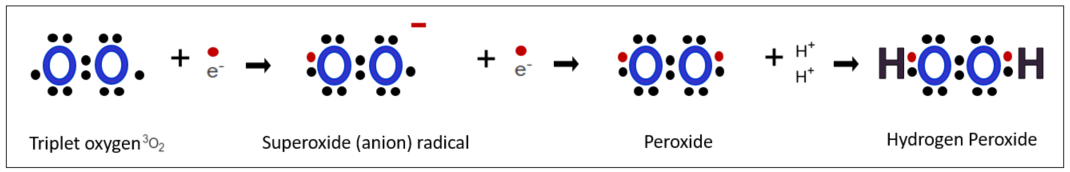
The birth of Hydrogen Peroxide
1. Formation of Peroxide
The superoxide radical acquires an additional electron from its surroundings, resulting in all the electrons in its orbitals forming pairs. This leads to the formation of a peroxide compound. Peroxides are a group of compounds with a structure of R-O-O-R, where organic groups(Rs) are synthesized with two oxygen atoms.
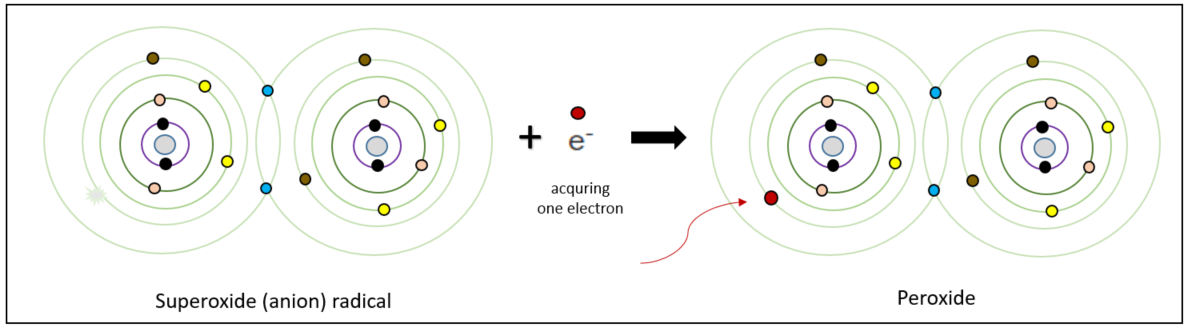
2. Hydrogen Peroxide
At this point, two hydrogen cations (also known as hydrogen protons since they lack electrons) without any electrons approach from the surroundings and attach to both sides. These hydrogen cations correspond to the organic group R. Technically, hydrogen peroxide is not a radical because all electron pairs, as can be seen in the diagram below, are paired. However, as we will see later, it still poses significant harm, so it is important to pay attention to radicals when dealing with hydrogen peroxide. Although hydrogen peroxide can be formed through other methods, for now, let's remember that it is formed from superoxide.
At this point, two hydrogen cations without electrons (also known as hydrogen protons because they are hydrogen ions without electrons) come from the surroundings and attach to both sides, corresponding to the organic matter R. Hydrogen peroxide is not a radical by definition because all of these electron pairs are paired, as you can see in the diagram below. However, it still poses significant harm in its own way as we will see in the future, we must pay attention to it when dealing with radicals. Hydrogen peroxide can also be produced in other ways, but here we must remember that hydrogen peroxide is formed from superoxide.
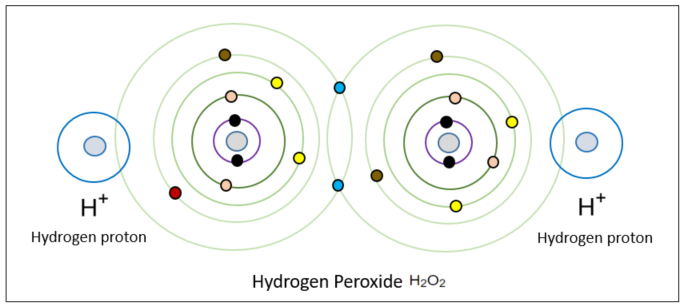
If we write the process of their formation using Lewis dot notation, it would be depicted as shown in the diagram below.
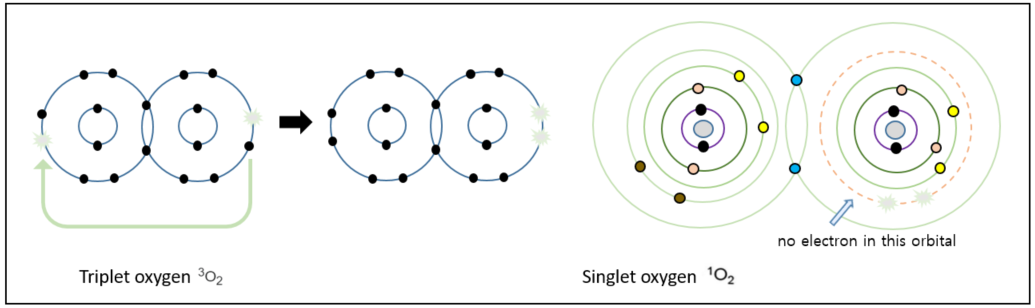
Singlet oxygen
When the most common form of oxygen we breathe, molecular oxygen (O2), gains an additional electron, it becomes the superoxide anion radical. We can observe that one of the oxygen atoms still has an unpaired electron in its orbital. However, in the case of molecular oxygen with a structure where each oxygen atom has one unpaired electron in its orbital, an external force can cause the lone electron on one oxygen atom to jump to the electron orbital of the other oxygen atoms, forming a pair there and completely making its original orbital empty. This is known as singlet oxygen. One possible reason for this unusual transformation is UV radiation.
In the beginning of this article, we saw that during cellular respiration, the energy generated through the transfer of electrons is used to push hydrogen protons out of the mitochondrial matrix into the intermembrane space. Considering the easy accessibility to individual electrons, abundance of hydrogen protons and oxygen, the inner membrane of the mitochondria provides an environment where not only superoxide but also hydrogen peroxide can be easily formed. It is estimated that the average human body has around 30 trillion cells [2]. Within a single cell, there are approximately 1,000 to 2,500 mitochondria, varying depending on the body part [3]. ATP production through cellular respiration accounts for 90% of the total ATP, and ATP, once produced, needs to be constantly generated as there is no way to store it. So, how much ATP is actually produced within the mitochondria in a day? Since a single cell uses an average of 10 billion ATP per day, the amount required for an adult required is said to be 3.0 × 10^25 ATP. Needless to say, these numbers also reflect the scale of possible electron leakage and loss inevitable in ATP production.
So far, we have looked at radicals related to oxygen. Now, in the next article, we will see how Hydrogen peroxide specifically gives rise to the most notorious and infamous Hydroxyl radical.
[References]
[1] Definition of radical
https://www.britannica.com/science/radical-chemistry
[2] Sender R, Fuchs S, Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4991899/
[3] Pizzorno J. Mitochondria-Fundamental to Life and Health.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4684129/
Mechanism of Superoxide and Hydrogen Peroxide Formation by Fumarate Reductase, Succinate Dehydrogenase, and Aspartate Oxidase
https://www.sciencedirect.com/science/article/pii/S0021925819718407#bib14



