Cyclization process of monosaccharides
In the previous article, we explored monosaccharides through their open chain structure. However, in reality, monosaccharides are known to be more stable when they have a cyclic structure rather than an open structure. From a biochemical perspective, this means that, considering that our human body is an aqueous environment both inside and outside the cells, monosaccharides almost always exist in a ring form in a physiological aqueous solution within the body. Let's take a closer look at how monosaccharides with a long chain-like carbon skeleton change into a round ring shape. Prior to this, it will be of great help in understanding the cyclization process if we point out some chemical characteristics that may be helpful in understanding the cyclization process.
In organic chemistry, the most important element is none other than carbon. However, the more I study organic chemistry, the more I come to think of oxygen as an essential supporting actor. Oxygen is an important element that forms functional groups in a wide variety of compounds and determines their reactivity. Oxygen appears in hydroxyl groups (-OH), carboxyl groups (-COOH), and compounds containing carbonyl groups (C=O) such as aldehydes, ketones, amides, esters, and in alkoxy groups (-OR) where oxygen is bonded to an alkyl group from which a hydrogen has been removed in alkanes. Its activity is remarkable. To understand the role oxygen plays in these various compounds, we must take a close look at its high electronegativity.
Electronegativity
Electronegativity is a relative value that indicates how strongly an atom in a covalent bond pulls shared electrons toward itself. It reflects how strongly the positively charged nucleus of an atom attracts electrons. For atoms that do not form covalent bonds (like He, Ne, Ar), electronegativity cannot be measured. The greater the number of protons in the nucleus, and the smaller the atomic radius (distance between nucleus and electrons), the stronger the pull of the nucleus on electrons, resulting in higher electronegativity. In the periodic table, considering the rows (periods) where atoms have the same number of electron shells, as you move from left to right, the number of protons in the nucleus increases, raising the atomic number. The increased positive charge strengthens the attraction to electrons and reduces atomic radius, resulting in higher electronegativity. For example, Carbon (C, 2.55) < Nitrogen (N, 3.04) < Oxygen (O, 3.44) < Fluorine (F, 3.98). In vertical columns (groups), as you move downward, the number of electron shells increases, enlarging atomic radius and weakening the nucleus’s pull on electrons, leading to lower electronegativity. Fluorine (F, 3.98) > Chlorine (Cl, 3.16) > Bromine (Br, 2.96) > Iodine (I, 2.66). Therefore, as seen in the figure below, fluorine, located at the top right, has the highest electronegativity. In fact, the typical ionic bond of a compound like NaCl can be seen as the ultimate result of an extreme difference in electronegativity.
Electronegativity and polarity of water
Electronegativity helps us understand how atoms in a covalent bond distribute the shared electron pair. A simple example is water (H₂O), composed of oxygen and hydrogen. As shown in the table above, oxygen (3.44) and hydrogen (2.2) have quite different electronegativities. In their covalent bond, the shared electron pair is much more heavily drawn toward oxygen. As a result, the oxygen, where more electrons gather, carries a partial negative charge, while the hydrogen, lacking electrons, carries a partial positive charge, thus creating a polarity. The oxygen with excess electrons becomes partially negative (δ⁻), and the hydrogen with fewer electrons becomes partially positive (δ⁺). The δ (delta) symbol is used to indicate an imbalance of charge due to differences in electronegativity between covalently bonded atoms. This difference in partial charges leads to weak electrostatic attraction known as hydrogen bonding. When another polar substance is attracted to the partial charges of a water molecule, we describe it as “dissolving in water.” For example, in salt (NaCl), the Na⁺ ion is drawn to the negatively charged oxygen (δ⁻) of water, and the Cl⁻ ion is drawn to the positively charged hydrogen (δ⁺), resulting in separation in water. When water boils, most hydrogen bonds break, and water molecules disperse into gas. Conversely, when water freezes, the molecules form stable hydrogen bonds in a hexagonal structure, turning into solid ice.
 |
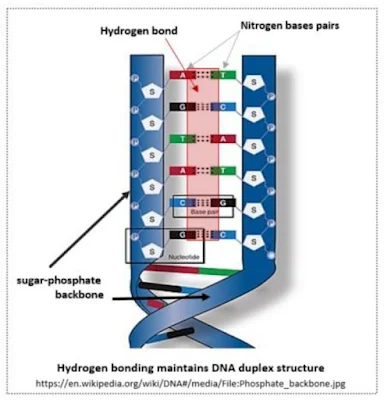
Hydrogen bonding that maintains DNA Double Helix
When hydrogen forms a covalent bond with an atom with high electronegativity, such as fluorine, oxygen, or nitrogen (F, O, N), the hydrogen, which is inferior(?) in its ability to pull electrons, acquires a partial positive charge and forms an electrostatic interaction, or hydrogen bond, with the surrounding partially negatively charged elements. Looking at the double helix structure of DNA, which contains our precious genetic information, the nitrogen and oxygen inside the nitrogen base pair located in the center have a partial negative charge (δ⁻) and form a hydrogen bond with hydrogen (δ⁺), which has a partial positive charge. Hydrogen bonds are weaker than covalent or ionic bonds, but they are strong enough to keep the DNA structure stable, and have an appropriate bond strength that allows them to be easily separated without any resistance when they need to be separated into two strands during DNA replication and transcription. It seems that the unique characteristic of hydrogen bonding is this exquisite and appropriate bond strength. If the double helix structure of DNA had not been maintained stably, mankind would not have been able to transmit genetic information and would not have even dreamed of evolution. Considering that the three-dimensional structure of proteins, which are the main materials of biomolecules, is maintained through hydrogen bonds between α-helices and β-sheets, we can begin to grasp the significance of electronegativity to living things. If water had not been formed of hydrogen bonds, the boiling and freezing points would have been much lower and most of the water would exist as a gas, thus making liquid water we know it today insufficient. Considering that life originated in water, this could have jeopardized the emergence of life itself.
Nucleophiles and Electrophiles
During the cyclization of monosaccharides, a chemical reaction called nucleophile attack occurs. To understand this, we must first learn about nucleophiles and electrophiles. Atoms or functional groups with lone pairs of electrons that can be shared with other atoms are called nucleophiles. In contrast, atoms that lack electrons are called electrophiles. The names literally mean “nucleus-loving” (nucleophile) and “electron-loving” (electrophile). Nucleophiles are likely to carry negative charges due to their high electron density and willingness to donate electron pairs to form covalent bonds. Electrophiles, with low electron density and a shortage of electrons, tend to carry positive charges and accept electrons. Both are in unstable, reactive states. Therefore, it can be easily predicted that a chemical reaction will naturally occur between those who have a lot and those who lack, between donors and acceptors. Categorizing reactants as nucleophiles or electrophiles helps explain and predict what kind of chemical reaction will take place. Since nucleophiles donate electron pairs, they act as Lewis bases, while electrophiles accept electron pairs and act as Lewis acids.
The reason for explaining the concepts of electronegativity and nucleophile and electrophile in great detail and length is to understand the cyclization process of monosaccharides. In theory, all monosaccharides are capable of cyclization. Just like connecting one end of a long linear chain to the other to create a necklace or bracelet, wouldn't it be possible to make a connection as long as the length is long enough? What we should focus on in the cyclization process is why and how it occurs, and what the results are. To understand why cyclization occurs, let’s talk more about oxygen.
The Ionization of Water – Oxygen at Work
Oxygen has an atomic number of 8. Its electron configuration shows 8 electrons, with 1s² in the first K shell and 2s² 2p⁴ in the second L shell. There are six valence electrons, with two participating in covalent bonds and two pairs remaining as lone pairs.
Since oxygen has high electronegativity, it carries a partial negative charge. When it finds a nearby atom or group with a partial positive charge due to lack of electrons, it attacks that area to form a covalent bond, thereby resolving the uneven distribution of electron density and reaching a more stable state. This reaction is called a “nucleophilic attack on an electrophile.” (Personally, I’ve always wondered why such a strong word like attack is used… perhaps it expresses the inescapable intensity of the interaction? 😅...) Previously, we mentioned that open-chain monosaccharides exist as cyclic forms in aqueous solutions almost 99% of the time. Considering that the human body is made up of 60–70% water, and that both inside and outside of cells are aqueous environments, let’s take a closer look.
Water consists of hydrogen and oxygen, but because highly electronegative oxygen strongly pulls electrons from hydrogen, hydrogen ends up in a positively charged state close to a bare proton (H⁺). This proton, lacking electrons and essentially just a nucleus, is a tiny particle. Nearby, oxygen atoms in water molecules still have two lone pairs of electrons not involved in bonding, and they generously donate one of these lone pairs to grab a hydrogen proton from another water molecule. Thus, a hydronium ion (H₃O⁺) is formed. The water molecule that lost a proton becomes a hydroxide ion (OH⁻). This process of forming these two ions is called the self-ionization of water, and since it happens continuously in both directions, hydronium and hydroxide ions are always present in small amounts in aqueous solutions.
 |
Hydronium ion (H₃O⁺) is a major ion that determines pH by substantially representing the number of protons in an aqueous solution. As the hydronium ion concentration increases, the pH decreases, creating a more acidic environment. Conversely, as the concentration of hydroxide ion (OH⁻) increases, the environment becomes more basic. pH not only affects acid-base reactions, but also serves as an important medium for promoting biochemical reactions in vivo. This is because the action of enzymes, protein structure, and ionic balance, which are essential for performing normal physiological functions of living organisms, all greatly depend on pH.
Hydronium ions (H₃O⁺) represent the actual number of protons in solution and determine the pH. As hydronium ion concentration increases, pH decreases, creating a more acidic environment. Conversely, as hydroxide ion (OH⁻) concentration increases, the environment becomes more basic. pH influences not only acid-base reactions but also serves as a key medium for biochemical reactions in living organisms. The activity of essential enzymes, the structure of proteins, and ionic balance—all depend heavily on pH. The proton that was lured by oxygen to become part of a hydronium ion can detach again and move to a nearby nucleophile. This phenomenon is called proton hopping. One might imagine that in the tug-of-war within water, the proton lives a very busy and engaged life, constantly being pulled around. For reference, unlike a typical covalent bond where both atoms equally contribute one electron each, a coordinate bond (or dative bond) is when one atom donates the entire electron pair. The bond between oxygen and hydrogen in a hydronium ion is an example. Since protons lack electrons, they cannot donate any, so the oxygen provides the full pair. The oxygen in hydroxide ions can also grab a proton from another water molecule and revert back to a neutral water molecule (H₂O), acting as a strong base ready to rapidly accept protons from acids. Now, let’s leave the battlefield-like world of water and return to monosaccharides. It seems we’ve laid enough groundwork to finally understand the cyclization of monosaccharides. In the next article, we’ll connect the chain into a ring in earnest.