Names to distinguish mirror-image twins
While it is understood that the two mirror images possess the properties of right-handedness and left-handedness, one might wonder what the basis is for designating them as right or left. It is important to distinguish between the two. However, these mirror-image isomers, which resemble twin siblings, are often challenging to differentiate due to their almost identical physical characteristics—such as boiling point, melting point, and solubility—as well as their chemical properties. In times when advanced techniques like X-ray crystallography did not exist, simpler methods, such as polarimetry, were used to distinguish and label these two twins.
Polarimetry and the rotational direction of enantiomers
In the early 19th century, French physicist Jean-Baptiste Biot observed that polarized light passing through solutions containing certain organic molecules would rotate its plane of polarization. Let’s examine the polarimetry method he used and the results of his experiments.
The light we commonly see is a type of electromagnetic wave where the electric field and magnetic field are perpendicular to each other. Since the electric field is relatively easier to manipulate, it is primarily explained in terms of the electric field. Generally, the electric field is evenly distributed in all directions, vibrating in all directions. However, devices such as polarizing filters can be used to restrict the electric field to vibrate in a specific direction, allowing it to oscillate in a particular plane while blocking all others. This method can be used to test optical activity. An organic molecule is placed in a sample tube, and polarized light is directed through it. If the sample contains chiral molecules, due to their asymmetric structure, the plane of polarization will rotate in one direction. On the other hand, if the molecules are achiral, the polarized light will not change direction as it passes through, resulting in no rotation. Only chiral molecules can rotate polarized light. When this rotation occurs, it is described as being "optically active." This optical activity is a unique characteristic of mirror-image isomers(enantiomer), which is why enantiomers are also referred to as "optical isomers."
Another interesting point is that the two enantiomers rotate the plane of polarization in opposite directions. While the angle of rotation for plane-polarized light is the same, the directions of rotation are opposite.
d(+) and l(-) notation
In the early 19th century, mirror-image isomers(enantiomers) that rotated polarized light to the right were designated as "dextrorotatory" or (+), while those that rotated it to the left were labeled as "levorotatory" or (-). If the light was observed to rotate in a clockwise direction, the corresponding enantiomer was classified as d or (+), while the opposite direction was classified as l or (-). At that time, relatively simple equipment could measure this rotation, making it a useful tool for distinguishing between the enantiomers without physical separation.
For reference, if equal amounts of both types of enantiomers are placed in a sample tube in a 1:1 ratio, they will cancel each other out, resulting in no rotation of the light. This mixture of equal amounts of enantiomers is known as a racemic mixture, or racemate. We will discuss racemic mixtures in more detail later.
D/L nomenclature
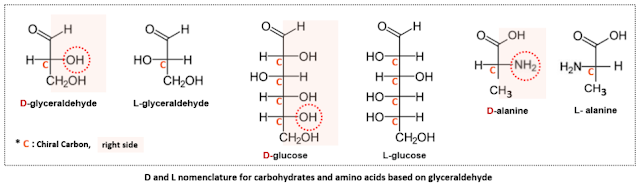
In cases of optical activity, the l, d or (+), (-) notation based on the direction of rotation of polarized light can provide names for the two enantiomers, but it does not explain the stereochemical arrangement of the molecules. Subsequently, Fischer projection was introduced to represent the three-dimensional structure of carbohydrates in a two-dimensional plane. The structure of glyceraldehyde, the simplest chiral molecule with only one chiral carbon, was expressed using Fischer projection, and this served as a reference for distinguishing between enantiomers. Specifically, the position of the hydroxyl group (OH) relative to the chiral carbon was used for differentiation: if the OH group was on the right, it was named D-glyceraldehyde, and if it was on the left, it was named L-glyceraldehyde.[1] The letters D and L are derived from the Latin words "dexter," meaning right, and "laevus," meaning left. This nomenclature for glyceraldehyde was standardized and subsequently applied to carbohydrates and amino acids, designating them as D or L forms.
In Fischer projection, the carbonyl group (C=O) is typically placed at the top for carbohydrates, while the carboxyl group (COOH) is positioned at the top for amino acids. When multiple chiral carbons are present, the chiral carbon furthest from these functional groups is used as a reference. If the hydroxyl group (OH) is on the right, it is designated as D, and if it is on the left, it is designated as L. For amino acids, if the amine group (NH₂) is on the right, it is classified as D. However, the D/L nomenclature has limitations in describing complex and large compounds, and it often caused confusion as it was not directly related to the earlier d, l distinction based on optical activity. For example, D-glucose exhibits (+) dextrorotation, while the same D-fructose shows (-) levorotation. As a result, this nomenclature has fallen out of widespread use, remaining only in limited contexts for certain biomolecules, such as carbohydrates (e.g., D-glucose). With the introduction of the R/S nomenclature, which provides a more systematic and clear distinction, the D/L nomenclature has naturally declined in use.
R/S nomenclature
Recently, the R/S nomenclature has been established as a set of rules for distinguishing the stereochemistry of molecules with one or more chiral centers, extending beyond just carbohydrates. This system, known as the Cahn-Ingold-Prelog (CIP) rules, is named after the three chemists who developed it and has become an international standard.
To determine whether a configuration is R or S, the first step involves assigning priorities to the four different substituents attached to the chiral center based on the atomic numbers of the atoms directly bonded to the chiral carbon. Generally, a higher atomic number corresponds to a higher atomic mass.
Next, the substituent with the lowest priority, which is typically hydrogen (H), is positioned at the back, away from the observer. The remaining three substituents are then arranged according to their priority. If the arrangement of these substituents from highest to lowest priority (1 → 2 → 3) appears to be in a clockwise direction, the configuration is designated as R (Rectus, meaning right). Conversely, if the arrangement is counterclockwise (1 → 2 → 3), it is designated as S (Sinister, meaning left).
This R/S nomenclature provides a clear and systematic way to describe the stereochemistry of chiral molecules, facilitating precise communication in the fields of chemistry and biochemistry.
The R/S nomenclature allows for a clear visual understanding of the stereochemical arrangement of molecules by prioritizing based on atomic numbers. This system provides objective and reproducible results, making it universally applicable to all types of molecules, including organic and inorganic compounds as well as biomolecules. Because it adheres to absolute rules without relying on comparisons with other molecules, the likelihood of errors is minimized. Most importantly, the International Union of Pure and Applied Chemistry (IUPAC) has officially approved this nomenclature as the standard, making it the naming convention used by chemists and biochemists worldwide.
Unlike the d(+)/l(-) nomenclature, which is based on the direction of optical activity observed experimentally, and the D/L nomenclature, which is based on the structural positions expressed in Fischer projections, the R/S nomenclature provides absolute stereochemical designations through the CIP rules. This nomenclature describes the absolute stereochemical arrangement within enantiomeric molecules, thus representing their absolute stereochemistry.
This theoretical nomenclature is reliable as it aligns closely with experimental results obtained through techniques such as X-ray crystallography, which visually confirms the spatial arrangement of atoms within a solid crystalline structure. However, it is important to note that experimental errors or interpretative differences can arise during analysis, particularly in situations where the stereochemistry influences reaction mechanisms, such as in drug synthesis and separation. Therefore, complementary methods, such as Nuclear Magnetic Resonance (NMR), should be used to re-confirm the structure. The necessity of accurately determining stereochemistry is crucial for predicting biochemical and pharmacological effects, a topic we will explore in more detail later.
Racemic mixture
As previously mentioned, while enantiomers exhibit optical activity by rotating light in opposite directions, when both enantiomers coexist, no net rotation occurs. This mixture is referred to as a racemic mixture or racemate.
One notable example is tartaric acid (H₂C₄H₄O₆), an organic acid found in grapes. During the fermentation process of grape juice into wine, tartaric acid naturally converts into its potassium salt, known as potassium acid tartarate (KC₄H₅O₆), which is also called cream of tartar. This occurs when one of the hydrogen ions in tartaric acid is replaced by a potassium ion.
Later, tartaric acid manufacturers inadvertently created a mysterious acid by refining potassium acid tartarate. This compound, which appeared to have the same chemical composition as tartaric acid, was named "racemic acid" after the Latin word "racemus," meaning "a bunch of grapes." Subsequently, Pasteur referred to it as para-tartaric acid.
The problem arose when, in 1832, Jean-Baptiste Biot discovered using a polarimeter that tartaric acid rotated polarized light to the right, indicating its optical activity. However, contrary to expectations, the racemic acid derived from tartaric acid exhibited no optical activity in the solution. This discrepancy highlighted the unique properties of racemic mixtures, where the optical activities of the enantiomers effectively cancel each other out.
To explain this intriguing situation, several chemists made efforts, but their interpretations were not entirely satisfactory. Pasteur decided to personally replicate the experiments of earlier crystallographers and crystallized a concentrated solution of sodium-ammonium tartrate at temperatures below 28 °C. As a result, he obtained two very different forms of crystals. Upon comparing these crystals, he found that they were mirror images of each other but could not be superimposed. The shapes of the crystals were hemihedral, meaning they only reflected half of the full symmetry elements, resulting in a special form where some faces of the crystal were missing or arranged asymmetrically.
When he examined the para-tartaric acid salt under a magnifying glass, he noticed that one set of half-faces was tilted to the left while the other was tilted to the right. He carefully separated the two groups of crystals using tweezers, ensuring to keep them aligned according to their respective orientations.
Upon testing the carefully separated crystals in solution using a polarimeter to observe any rotation of polarized light, he found, surprisingly, that the crystals with left-tilted faces rotated the light to the left, while those with right-tilted faces rotated the light to the right. This indicated that they exhibited optical activity. Even more astonishing was the fact that when he mixed equal weights of the two types of crystals to create a solution, the resulting mixture showed a neutral reaction with no rotation of light, as the two directions effectively canceled each other out.
Pasteur not only physically separated the two enantiomers through this experiment but also became the first to demonstrate the principle that their equal mixing results in no optical activity. Furthermore, the findings significantly advanced the understanding of the three-dimensional structure and properties of molecules, marking an important milestone in the development of stereochemistry. The inference that chiral structures could arise from the asymmetry of molecules was made, leading to the discovery of the principles of molecular asymmetry, which became a crucial first step in understanding the three-dimensional characteristics of molecular structures.
Even after this experiment, Pasteur continued his research on asymmetry and made significant contributions to the early development of stereochemistry through studies on partial stereoisomerism and the separation of racemic mixtures using partially stereoisomeric crystals. The concept of racemic mixtures, which he described, is particularly important in life sciences, and we will revisit this in connection with homochirality.
“In the fields of observation, chance favors only the prepared mind.”
Here, I would like to express my admiration for Pasteur. In fact, I first learned about this scientist while studying immunology. He proved that microorganisms are the cause of diseases and discovered methods to sterilize them at low temperatures, paving the way for countless children to safely consume milk. He also contributed to immunology by developing vaccines for rabies and anthrax using weakened viruses or pathogens to induce immune responses. Known as the father of bacteriology, he famously said during a university lecture, “In the fields of observation, chance favors only the prepared mind.” This quote is widely recognized and often cited as good advice for life, capturing the essence of Pasteur, who had remarkable observational skills.
The "prepared mind" mentioned here encompasses the ability to recognize the unusual, think critically, imagine interpretations, and take risks that might lead to failure while being willing to explore new avenues beyond the original plan. I believe that serendipitous discoveries in science reflect an interesting interaction between scientific observation and chance. Was it merely a coincidence that Pasteur used sodium-ammonium tartrate in his experiments? The conglomerate form, where two types of enantiomers exist as separate crystalline entities within a racemic mixture, is extremely rare, occurring in less than 10% of cases, and sodium-ammonium tartrate was one of them. The term "conglomerate" in chemistry refers to a special form of a racemic mixture where the R and S enantiomers exist as physically separated crystalline states. In contrast, when the R and S enantiomers are contained within the same crystal structure, it is referred to as a racemate. The fact that these crystals could be visually distinguished and separated using tweezers was certainly a stroke of genius. Pasteur also emphasized the importance of conducting the experiment in the morning, as rising temperatures during the day could partially dissolve the crystals and eliminate the hemihedral faces. Indeed, sodium-ammonium tartrate crystallizes in a conglomerate form at 20 °C, but dehydration begins at 28 °C, and by 30 °C, it transforms into a racemate.[2]
Whether these critical details were purely coincidental remains unknown, but I have no doubt that he always possessed a prepared mind (after all, I am a fan of his).
Let’s explore what chirality means in relation to our biological systems in the following article.
[References]
[1] Biochemistry, Mary Campbell, Shawn O. Farrell, Owen McDougal. Ninth Edition
[2] Pasteur and chirality: A story of how serendipity favors the prepared minds
https://doi.org/10.1002/chir.23349
Pasteur's Resolution of Racemic Acid: A Sesquicentennial Retrospect and a New Translation